��Ŀ����
��18�֣�I��ʵ�����Ʊ����ռ������SO2�������������¡�
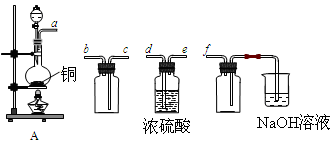
(1)װ��A����SO2���������������Ӹ������ӿڣ�˳��Ϊa�� ____�� ____ ��____��____f��
(2)װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ____________________________________��
(3)��֤������������SO2�ķ�����________________________________________��
II��SO2����Ϊ��ɫ���壬��ǿ�Ҵ̼�����ζ��������Ҫ��Ⱦ��֮һ������һ���Ļ�ԭ�ԣ�̽��SO2���廹ԭFe3����I2����ʹ�õ�ҩƷ��װ����ͼ��ʾ��
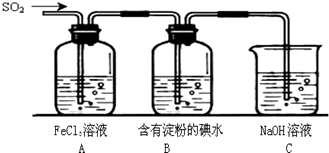
(1)װ��A�е�������__________����SO2��ԭFe3���ķ�Ӧ��SO2��Fe3�������ʵ���֮����_______��
(2)װ��C��������____________________________________��
(3)��Ҫ��A�е�FeCl3��Һ����ȡ���壬������е�ʵ��������裺����Ũ������ȴ�ᾧ�����ˣ�����һϵ�в�����û���õ��IJ���������( )
(4)������װ����ͨ�������SO2��Ϊ����֤A��SO2��Fe3��������������ԭ��Ӧ��ȡA�е���Һ���ֳ����ݣ������������ʵ�飺
�����٣�����һ����Һ�м�����������KMnO4��Һ���Ϻ�ɫ��ȥ��
�����ڣ����ڶ�����Һ�м���KSCN��Һ������죬�ټ������Ƶ���ˮ����Һ��졣
������������������______��ԭ����___________________________________��
(5)�ܱ����Ļ�ԭ��������SO2��������________________________________________��

(1)װ��A����SO2���������������Ӹ������ӿڣ�˳��Ϊa�� ____�� ____ ��____��____f��
(2)װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ____________________________________��
(3)��֤������������SO2�ķ�����________________________________________��
II��SO2����Ϊ��ɫ���壬��ǿ�Ҵ̼�����ζ��������Ҫ��Ⱦ��֮һ������һ���Ļ�ԭ�ԣ�̽��SO2���廹ԭFe3����I2����ʹ�õ�ҩƷ��װ����ͼ��ʾ��

(1)װ��A�е�������__________����SO2��ԭFe3���ķ�Ӧ��SO2��Fe3�������ʵ���֮����_______��
(2)װ��C��������____________________________________��
(3)��Ҫ��A�е�FeCl3��Һ����ȡ���壬������е�ʵ��������裺����Ũ������ȴ�ᾧ�����ˣ�����һϵ�в�����û���õ��IJ���������( )
| A����ƿ | B���ƾ��� | C��©�� | D���ձ� E�������� |
�����٣�����һ����Һ�м�����������KMnO4��Һ���Ϻ�ɫ��ȥ��
�����ڣ����ڶ�����Һ�м���KSCN��Һ������죬�ټ������Ƶ���ˮ����Һ��졣
������������������______��ԭ����___________________________________��
(5)�ܱ����Ļ�ԭ��������SO2��������________________________________________��
I.��1��d��e��c��b ��2��Cu��2H2SO4(Ũ)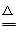 CuSO4��2H2O��SO2��
CuSO4��2H2O��SO2��
��3��������ͨ��Ʒ����Һ����Һ��ɫ�����Ȼָ�ԭɫ
��.��1����Һ��ɫ�ɻ�ɫ��Ϊdz��ɫ��1:2
��2������SO2����ֹSO2��Ⱦ������ ��3��A
��4���٣� SO2������SO2��ʹ����KMnO4��Һ��ɫ ��5��B����ɫ��ȥ
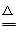 CuSO4��2H2O��SO2��
CuSO4��2H2O��SO2����3��������ͨ��Ʒ����Һ����Һ��ɫ�����Ȼָ�ԭɫ
��.��1����Һ��ɫ�ɻ�ɫ��Ϊdz��ɫ��1:2
��2������SO2����ֹSO2��Ⱦ������ ��3��A
��4���٣� SO2������SO2��ʹ����KMnO4��Һ��ɫ ��5��B����ɫ��ȥ
���������I.��1��SO2�ܶȱȿ������������ſ������ռ����ռ�ǰ��ҪŨ�����������Ҫ������������Һ���գ������ȷ������˳��Ϊa��d��e��c��b��f��
��2��Ũ�������ǿ�����ԣ��ڼ��ȵ���������ͭ����������ԭ��Ӧ����Ӧ�Ļ�ѧ����ʽΪCu��2H2SO4(Ũ)
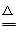 CuSO4��2H2O��SO2����
CuSO4��2H2O��SO2������3��SO2����Ư���ԣ���˼���SO2�ķ����ǣ�������ͨ��Ʒ����Һ����Һ��ɫ�����Ȼָ�ԭɫ��
��.��1���Ȼ������������ԣ��ܰ�SO2�����������ᣬ�������ӱ���ԭΪ�������ӣ����װ��A�е�ʵ�������ǣ���Һ��ɫ�ɻ�ɫ��Ϊdz��ɫ��A�з�Ӧ�����ӷ���ʽΪ2Fe3++SO2+2H2O��2Fe2++SO42-+4H+����˲μӷ�Ӧ��SO2��Fe3+�����ʵ���֮�ȵ��ڼ�����֮�ȣ���Ϊ1:2��
��2������������������������д̼�����ζ��ֱ���ŷ���Ⱦ�������ܺͼӦ�����κ�ˮ�����Կ��ü�Һ�����������ʴ�Ϊ��
��3������ʹ�������������ƾ��ơ�������������ʹ��������©�����ձ��������������û���õ�����������ƿ����ѡA��
��4��Fe2+ʹ���������Һ��ɫ����ʵ����SO2�ǹ����ģ������������л�ԭ�ԣ����������ǿ�����ԣ���������Ҳ���������ط���������ԭ��Ӧʹ���������Һ��ɫ����˷����ٲ�������
��5�����ݷ�ӦI2+SO2+2H2O��2HI+H2SO4��֪������������ʹ���е�ĵ�����Һ��ɫ��˵��������������ԭ��Ӧ�����������������������ǻ�ԭ������ԭ���Ļ�ԭ�Դ��ڻ�ԭ����Ļ�ԭ�ԣ�����ܱ����Ļ�ԭ��������SO2��������B����ɫ��Һ��ɫ��2���Ʊ�������ʵ��̽����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ