��Ŀ����
�����£���һԪ��HA��Һ��NaOH��Һ�������ϣ�ʵ�����������
| ʵ���� | ��ʼŨ��c(HA) | ��ʼŨ��c(NaOH) | ��Ӧ����Һ��pH |
| �� | 0.1 mol��L��1 | 0.1 mol��L��1 | 9 |
| �� | x | 0.2mol��L��1 | 7 |
����˵����ȷ����
A��ʵ��ٷ�ӦǰHA��Һ��c(H+)��c(OH��)+ c(A��)
B��ʵ��ٷ�Ӧ����Һ��c(A��)��c(Na +)
C��ʵ��ڷ�ӦǰHA��ҺŨ��x��0.2 mol��L��1
D��ʵ��ڷ�Ӧ����Һ��c(A��)+ c(HA)= c(Na+)
AC
�������������A����Ӧǰ��HA������� ����H+��A����������Һ�е�H+����HA��������ĺ�ˮ��������ģ���ˮ���������H+��OH����ȣ����Է�ӦǰHA��Һ��c(H+)��c(OH��)+ c(A��)����ȷ��B��ʵ��ٷ�Ӧ����ҺΪNaA��Һ������A��ˮ��ʹ��Һ�ʼ��ԣ�����c(A��)<c(Na +)������C�����������Ũ�ȵ�����Ӧ�����ҺΪ���ԣ���ʹ��Һ�����ԣ�������ʵ���Ӧ���ڼ����x��0.2 mol��L��1����ȷ��D��ʵ��ڷ�Ӧ����Һ�����ԣ����ݵ���غ㣬��c(A��)= c(Na+)������ѡAC��
���㣺�����ᡢ����Һ�ļ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д����������У�����������ʵ��ǣ� ��
| A����ˮ | B�������� | C���ƾ� | D�����ᱵ |
25��ʱ����c(CH3COOH)��c(CH3COO��)��0.1 mol��L��1��һ����ᡢ�����ƻ����Һ����Һ��c(CH3COOH)��c(CH3COO��)��pH �Ĺ�ϵ��ͼ��ʾ�������й���Һ������Ũ�ȹ�ϵ��������ȷ����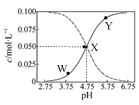
| A��Y������ʾ����Һ�У�c(CH3COO��)��c(CH3COOH)��c(H��)��c(OH��) |
| B��W������ʾ����Һ�У�c(Na��)��c(H��)��c(OH��)��c(CH3COOH)��0.1mol��L��1 |
| C�����¶��´���ĵ���ƽ�ⳣ��Ϊ10��4.75 mol��L��1 |
| D����X������ʾ����Һ�м���������0.05 mol��L��1 NaOH��Һ ��c(H��)��c(CH3COOH)��c(OH��) |
���и���ʵ��������������ó��Ľ�����ȷ����
| ѡ�� | ʵ����� | ʵ������ | �� �� |
| A | ������Fe(NO3)2������ˮ�ܽ⣬�μ�ϡH2SO4�ữ���ٵμ�KSCN��Һ | ��Һ��ɺ�ɫ | Fe(NO3)2�����ѱ��� |
| B | ������ij��ɫ����ͨ�����ʯ��ˮ | ���ְ�ɫ���� | ������һ����CO2 |
| C | �ֱ�ⶨ������0.1 mol��L��1 Na2SiO3��Һ��Na2CO3��Һ��pH | pH��Na2SiO3�� Na2CO3 | �ǽ����ԣ�Si��C |
| D | ��Ũ�Ⱦ�Ϊ0.1 mol��L��1 NaCl��NaI�����Һ�У��μ�����AgNO3��Һ | ���ֻ�ɫ���� | Ksp(AgCl)��Ksp(AgI) |
�����й��ܶȻ�����Ksp��˵����ȷ����
| A�������£���BaCO3������Һ�м���Na2CO3���壬BaCO3��Ksp��С |
| B���ܶȻ�����Kspֻ���¶�Ӱ�죬�¶�����Ksp��С |
| C���ܶȻ�����Kspֻ���¶�Ӱ�죬�¶�����Ksp���� |
| D�������£���Mg(OH)2������Һ�м���NaOH���壬Mg(OH)2��Ksp���� |
�����£���20 mL 0.2 mol/L H2A��Һ�еμ�0.2 mol/L NaOH��Һ���й����ӵ����ʵ����仯��ͼ(���Т����H2A�������HA���������A2��)������ͼʾ�жϣ�����˵����ȷ����
| A����V[NaOH(aq)]��20 mLʱ����Һ������Ũ�ȴ�С��ϵ�� c(Na��)>c(HA��)>c(H��)>c(A2��)>c(OH��) |
| B���������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����Һ��ˮ�ĵ���̶ȱȴ�ˮ�� |
| C��H2A��һ������ķ���ʽΪH2A===HA����H�� |
| D����NaHA��Һ����ˮϡ�͵Ĺ����У�pH��������Ҳ���ܼ�С |
ijѧ���ü�ʽ�ζ�����ȡ0.1 mol��L��1��NaOH��Һ����ʼʱ����Һ�����Ϊ1.00 mL��ȡ��������Һ����Һ�棬����Ϊ11.00 mL����ͬѧ�ڲ�����ʵ��ȡ����Һ�����Ϊ( )
| A������10.00 mL | B����10.00 mL |
| C������10.00 mL | D������11.00 mL |
25��ʱ����Ũ��Ϊ0.1000 mol��L��1��NaOH��Һ�ζ�20.00 mLŨ�Ⱦ�Ϊ0.1000 mol��L��1��������HX��HY��HZ���ζ���������ͼ��ʾ������˵����ȷ����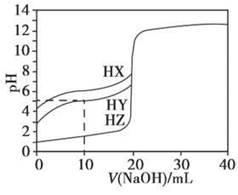
| A������ͬ�¶��£�ͬŨ�ȵ���������Һ�ĵ�������˳��HZ��HY��HX |
| B�����ݵζ����ߣ��ɵ�Ka(HY)��10��5 |
| C��������HX��HY��Һ�������Ϻ���NaOH��Һ�ζ���HXǡ����ȫ��Ӧʱ��c(X��)��c(Y��)��c(OH��)��c(H��) |
D��HY��HZ��ϣ��ﵽƽ��ʱ��c(H��)�� ��c(Z��)��c(OH��) ��c(Z��)��c(OH��) |