��Ŀ����
ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�

���ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������140����ˮ�������ѣ�
����������������Ҵ��Ʊ�1��2-���������װ����ͼ��ʾ��

�й������б����£�
�ش��������⣺
��1���ڴ��Ʊ�ʵ���У�Ҫ������Ѹ�ٵذѷ�Ӧ�¶���ߵ�170�����ң�������ҪĿ����
a��������Ӧ b���ӿ췴Ӧ�ٶ� c����ֹ�Ҵ��ӷ� d�����ٸ�������������
��2����װ��C��Ӧ����
a��ˮ b��Ũ���� c������������Һ d������̼��������Һ
��3���жϸ��Ʊ���Ӧ�Ѿ��������������
��4����1��2-��������ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã�����Ӧ��
��5����������������δ��Ӧ��Br2�������
a��ˮb������������Һc���⻯����Һd���Ҵ�
��6�������������������������ѣ�����
��7����Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ����

���ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������140����ˮ�������ѣ�
����������������Ҵ��Ʊ�1��2-���������װ����ͼ��ʾ��

�й������б����£�
| �Ҵ� | 1��2-�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g?cm-3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 | 132 | 34.6 |
| �۵�/�� | -130 | 9 | -116 |
��1���ڴ��Ʊ�ʵ���У�Ҫ������Ѹ�ٵذѷ�Ӧ�¶���ߵ�170�����ң�������ҪĿ����
d
d
��������ȷѡ��ǰ����ĸ��a��������Ӧ b���ӿ췴Ӧ�ٶ� c����ֹ�Ҵ��ӷ� d�����ٸ�������������
��2����װ��C��Ӧ����
c
c
����Ŀ�������շ�Ӧ�п������ɵ��������壻������ȷѡ��ǰ����ĸ��a��ˮ b��Ũ���� c������������Һ d������̼��������Һ
��3���жϸ��Ʊ���Ӧ�Ѿ��������������
�����ɫ��ȫ��ȥ
�����ɫ��ȫ��ȥ
����4����1��2-��������ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã�����Ӧ��
��
��
�㣨��ϡ������¡�������5����������������δ��Ӧ��Br2�������
b
b
ϴ�ӳ�ȥ��������ȷѡ��ǰ����ĸ��a��ˮb������������Һc���⻯����Һd���Ҵ�
��6�������������������������ѣ�����
����
����
�ķ�����ȥ����7����Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ����
��ϩ���巴Ӧʱ���ȣ���ȴ�ɱ�����Ĵ����ӷ�
��ϩ���巴Ӧʱ���ȣ���ȴ�ɱ�����Ĵ����ӷ�
�����ֲ��ܹ�����ȴ�����ñ�ˮ������ԭ����1��2-������������̵�ϵͣ�9�棩��������ȴ��ʹ�����̶�ʹ��·����
1��2-������������̵�ϵͣ�9�棩��������ȴ��ʹ�����̶�ʹ��·����
����������1���Ҵ���Ũ����140��������·������Ӽ���ˮ��
��2��Ũ�������ǿ�����ԣ����������Ҵ��е�̼��
��3����ϩ����ˮ�����˼ӳɷ�Ӧ��
��4������1��2-���������ˮ���ܶ���Դ�С���
��5��Br2���Ժ��������Ʒ���������ԭ��Ӧ��
��6������1��2-�������������ѵķе㲻ͬ���н��
��7�����ӷ����÷�Ӧ���ȣ�
��2��Ũ�������ǿ�����ԣ����������Ҵ��е�̼��
��3����ϩ����ˮ�����˼ӳɷ�Ӧ��
��4������1��2-���������ˮ���ܶ���Դ�С���
��5��Br2���Ժ��������Ʒ���������ԭ��Ӧ��
��6������1��2-�������������ѵķе㲻ͬ���н��
��7�����ӷ����÷�Ӧ���ȣ�
����⣺��1���Ҵ���Ũ����140��������£�������������ˮ���������ѣ��ʴ�Ϊ��d��
��2��Ũ�������ǿ�����ԣ����Ҵ������ɶ�����̼����������ԭ�ɶ�����������̼�����������ܺ�����������Һ��Ӧ���ʴ�Ϊ��c��
��3����ϩ����ˮ�����ӳɷ�Ӧ����1��2-�������飬1��2-��������Ϊ��ɫ���ʴ�Ϊ�������ɫ��ȫ��ȥ��
��4��1��2-���������ˮ�����ܣ�1��2-���������ܶȱ�ˮ�ʴ�Ϊ���£�
��5��������Br2���������Ʒ�����Ӧ��2NaOH+Br2�TNaBr+NaBrO+H2O���ʴ�Ϊ��b��
��6��1��2-�������������ѵķе㲻ͬ�����߾�Ϊ�л�����ܣ�������ķ��������Ƿ��룬�ʴ�Ϊ������
��7�����ڳ����£��ӷ�����ϩ���巴Ӧʱ���ȣ�����ӷ�����ȴ�ɱ�����Ĵ����ӷ�����1��2-������������̵�9��ϵͣ����ܹ�����ȴ��
�ʴ�Ϊ����ϩ���巴Ӧʱ���ȣ���ȴ�ɱ�����Ĵ����ӷ���1��2-������������̵�ϵͣ�9�棩��������ȴ��ʹ�����̶�ʹ��·������
��2��Ũ�������ǿ�����ԣ����Ҵ������ɶ�����̼����������ԭ�ɶ�����������̼�����������ܺ�����������Һ��Ӧ���ʴ�Ϊ��c��
��3����ϩ����ˮ�����ӳɷ�Ӧ����1��2-�������飬1��2-��������Ϊ��ɫ���ʴ�Ϊ�������ɫ��ȫ��ȥ��
��4��1��2-���������ˮ�����ܣ�1��2-���������ܶȱ�ˮ�ʴ�Ϊ���£�
��5��������Br2���������Ʒ�����Ӧ��2NaOH+Br2�TNaBr+NaBrO+H2O���ʴ�Ϊ��b��
��6��1��2-�������������ѵķе㲻ͬ�����߾�Ϊ�л�����ܣ�������ķ��������Ƿ��룬�ʴ�Ϊ������
��7�����ڳ����£��ӷ�����ϩ���巴Ӧʱ���ȣ�����ӷ�����ȴ�ɱ�����Ĵ����ӷ�����1��2-������������̵�9��ϵͣ����ܹ�����ȴ��
�ʴ�Ϊ����ϩ���巴Ӧʱ���ȣ���ȴ�ɱ�����Ĵ����ӷ���1��2-������������̵�ϵͣ�9�棩��������ȴ��ʹ�����̶�ʹ��·������
�����������Ϊ�ۺϣ���Ҫ�������Ҵ��Ʊ�1��2-�������飬����������ʵĻ�����ѧ���ʣ��ǽ����Ĺؼ���ƽʱ��ע�������ط�Ӧ֪ʶ���Ѷ��еȣ�

��ϰ��ϵ�д�
 ����������������ϵ�д�
����������������ϵ�д�
�����Ŀ
��ͼ��ʵ�����Ʊ�1��2-�������鲢����һϵ�����ʵ���װ�ã����ȼ��г��豸���ԣ���
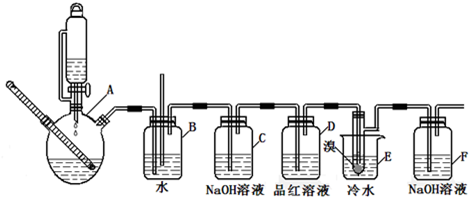
�����������
��1��A��ҩƷΪ1��3����ˮ�Ҵ���Ũ������Һ��д���Ʊ���ϩ�Ļ�ѧ��Ӧ����ʽ�� ��
��2�����巢��װ��ʹ����ͨ��Һ©����ԭ�� ��
��3��װ��D��Ʒ����Һ�������� ��ͬʱBװ���ǰ�ȫƿ�����ʵ�����ʱE���Ƿ�����������д������ʱ������ ��
��4����Ӧ������Ӧ����ˮ��ȴװ��E������ҪĿ���� �����ֲ��ܹ�����ȴ�����ñ�ˮ������ԭ���� ��
��5���жϸ��Ʊ���Ӧ�Ѿ������ķ����� �����ѧ�����ַ�Ӧ����ʱ����ˮ�Ҵ��������������ֵ����ԭ���� ��
��6����ѧ�������װ��F�пɸ������������Ȼ�̼Һ�����ն�������壬�жϸ������Ȼ�̼Һ���Ƿ���� ����ǡ�������ԭ���� ��

�����������
| �Ҵ� | 1��2-�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g/cm3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 | 132 | 34.6 |
| �۵�/�� | һl30 | 9 | -1l6 |
��2�����巢��װ��ʹ����ͨ��Һ©����ԭ��
��3��װ��D��Ʒ����Һ��������
��4����Ӧ������Ӧ����ˮ��ȴװ��E������ҪĿ����
��5���жϸ��Ʊ���Ӧ�Ѿ������ķ�����
��6����ѧ�������װ��F�пɸ������������Ȼ�̼Һ�����ն�������壬�жϸ������Ȼ�̼Һ���Ƿ����
��֪ʵ�����Ʊ�1��2-����������ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������l40����ˮ�������ѡ�������������������Ҵ��Ʊ�1,2�����������װ������ͼ��ʾ��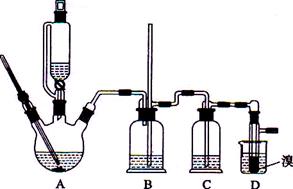
�й������б����£�
| | �Ҵ� | 1,2-�������� | ���� |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶȣ�g �� cm-3 | 0.79 | 2.2 | 0.71 |
| �е㣯�� | 78.5 | 132 | 34.6 |
| �۵㣯�� | һl30 | 9 | -1l6 |
(1)��Ӧԭ����___________________________________________________________
(2)��װ��C��Ӧ���� ��(����ȷѡ��ǰ����ĸ)��Ŀ����_______________
a��ˮ b��Ũ���� c������������Һ d������̼��������Һ
(3)�жϸ��Ƹ���Ӧ�Ѿ�������������� ��
(4)�����������������������ѣ����� �ķ�����ȥ��
(5)��Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ���� �����ֲ��ܹ�����ȴ(���ñ�ˮ)����ԭ���� ��
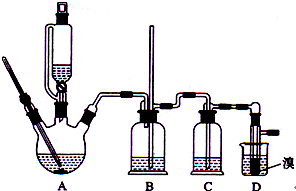 ��2012?���ϣ�ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�
��2012?���ϣ�ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£� ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�
ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�