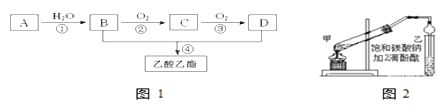
��Ŀ����
����Ŀ��X�Ǻϳ�̼���������һ����Ч��������ԭ������������EDTA��Fe3+��Ӧ�õ���
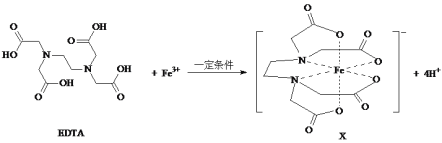
��1��EDTA��̼ԭ���ӻ��������Ϊ_________________��EDTA������Ԫ�صĵ縺����С�����˳��Ϊ__________________��
��2��Fe3+��̬��������Ų�ʽΪ_________________��
��3��EDTA������ʮһ�����Է��������dz��ӽ�����EDTA�ķе㣨540.6 �棩������ʮһ��ķе㣨100 �棩�ߵ�ԭ����_________��
��4��1 mol EDTA�к��ЦҼ�����ĿΪ______________��
��5��X�е���λԭ����___________��
���𰸡�sp2��sp3 H < C < N < O [Ar]3d5��1s22s22p63s23p63d5 EDTA���Ӽ������� 35 mol N��O
��������
��1��EDTA���Ȼ���Cԭ�Ӽ۲���ӶԸ�����3���Ǽ���Cԭ�Ӽ۲���ӶԸ�����4�����ݼ۲���ӶԻ��������ж�Cԭ���ӻ�������ͣ�Ԫ�صķǽ�����Խǿ����縺��Խ��
��2��Feԭ��ʧȥ3���������������ӣ����ݹ���ԭ����д�����Ӻ�������Ų�ʽ��
��3�����γɷ��Ӽ�����������۷е�ϸߣ�
��4�����۵���Ϊ����������˫���к���һ��������һ����������1��EDTA��������������Ŀ35��
��5��X��N��Oԭ���ṩ�µ��Ӷԡ�
(1)EDTA���Ȼ���Cԭ�Ӽ۲���ӶԸ�����3���Ǽ���Cԭ�Ӽ۲���ӶԸ�����4,���ݼ۲���ӶԻ��������ж�Cԭ���ӻ��������,ǰ��Ϊsp2�ӻ�������Ϊsp3�ӻ���Ԫ�صķǽ�����Խǿ����縺��Խ�ǽ�����H<C<N<O����縺��H<C<N<O��
�ʴ�Ϊ��sp2��sp3��H<C<N<O��
(2)Feԭ��ʧȥ3����������������,���ݹ���ԭ����д�����Ӻ�������Ų�ʽΪ[Ar]3d5��1s22s22p63s23p63d5��
�ʴ�Ϊ��[Ar]3d5��1s22s22p63s23p63d5��
(3)���γɷ��Ӽ�����������۷е�ϸߣ�EDTA���γɷ��Ӽ�������������۷е�ϸߣ�
�ʴ�Ϊ��EDTA���Ӽ���������
(4)���۵���Ϊ����������˫���к���һ��������һ����������1��EDTA��������������Ŀ35������1molEDTA�к�����������ĿΪ35mol��
�ʴ�Ϊ��35mol��
(5)X��N��Oԭ���ṩ�µ��Ӷԣ�����N��OΪ��λԭ�ӣ�
�ʴ�Ϊ��N��O��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���Ʊ��Ʋ�������Fe3O4����ģ��������ˮ����Ԫ�ص���Ҫʵ���������£�

��֪����CaO2��������Һ�е�FeCl2����Ӧ����Fe(OH)3��Fe3����
�ڲ��ӵ�Ca2��Ƕ��Fe3O4�У�ϴ��ʱ����ʧ������ʱ���γ�Ca3(PO4)2�ȳ�����
����Һ��pH���������������������Ӱ�졣pHԽ�ߣ��������������Խ�ࣻ pHԽ�ͣ��������������Խ�ࡣ
��1����FeCl2��FeCl3�����Һ�еμ�NaOH��Һ��һ�������·�Ӧ����Fe3O4�������ӷ���ʽΪ___________��
��2����������pH��11������������70�������½��У����˵ļ��ȷ�ʽΪ________��Ϊ��߹�����Ч�������ɲ�ȡ�Ĵ�ʩΪ_______________��
��3����Ԫ�ص�����Ч����H3PO4ˮ��Һ�к������ֲַ�������pH�Ĺ�ϵ�ֱ���ͼ1��ͼ2��ʾ��
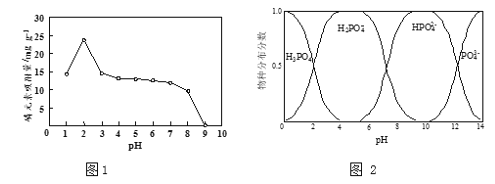
�ٲ������KH2PO4��Һģ���ˮ���������������(pH > 2)����Ԫ���������ϴ�ԭ���ǣ�pHԽ�ͣ��������������������Խ�࣬���������������ӣ�___________________
�ڲ������������ȡ�����ü�Һ���������ס���ϱ������ݣ������Ʋ�������Fe3O4������������������������ȵ������У�________��
��ͬ���������������������������Ƚ�
������ | ����Ʒ | ��Fe3O4 | �մɲ��� |
������/mg��g��1 | 24.1 | 5.0 | 12.5 |
��4������ƴӲ����Ӧ���������ƿ�л�ȡ�Ʋ�������Fe3O4��Ʒ��ʵ�鷽�����ô��������������Һ���룬______________����ɸ��ɸ�ֵõ���Ʒ (ʵ������ʹ�����Լ��������У�����ˮ����ˮ�Ҵ���pH�ơ��в�������)��