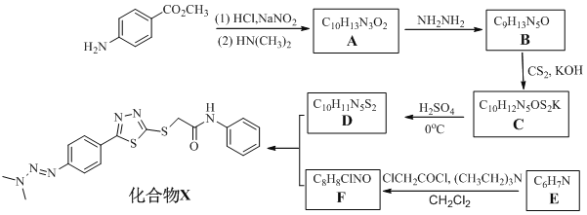
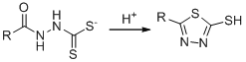��Ŀ����
����Ŀ���绯ѧ�����������ж�������Ҫ�����ú����塣
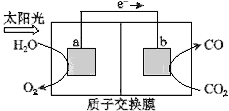
(1)ͼ1Ϊ��ɫ��Դ��������(CH3OCH3)ȼ�ϵ�����Ĺ���ԭ��ʾ��ͼ���õ�صĸ�����ӦʽΪ_________��

(2)�Ʊ����������ƣ�Na2S2O5���ɲ�������Ĥ��⼼����װ����ͼ2��ʾ������SO2������Һ�к���NaHSO3��Na2SO3�������ĵ缫��ӦʽΪ_______������____�ҵ�NaHSO3Ũ�����ӡ���������Һ���нᾧ��ˮ���ɵõ�Na2S2O5��
(3)�������ȣ�ClO2������ɫ������ˮ�����壩�Ǹ�Ч���Ͷ������������ش��������⣺
ʵ������NH4Cl�����ᡢNaClO2(�������ƣ�Ϊԭ�ϣ�ͨ�����¹����Ʊ�ClO2�����ʱ������Ӧ�Ļ�ѧ����ʽΪ________________����ҺX�д������ڵ���������________________��

(4)�ⶨ�������ClO2�ĺ�����
��.����ƿ�м��������ĵ⻯�أ���50 mLˮ�ܽ���ټ���3 mLϡ���ᡣ��һ�����Ļ������ͨ������Һ�г�����ա�
��.��0.1000 mol��L-1��������Ʊ���Һ�ζ���ƿ�е���Һ��I2+2S2O32-��2I��+S4O62-)���Ե�����ҺΪָʾ����ʾ�յ�ʱ����ȥ20.00 mL�����������Һ��
����ƿ��ClO2��⻯�ط�Ӧ�����ӷ���ʽΪ__________________��
�ڲ�û������ClO2������Ϊ______��
�۲ⶨ�������ClO2�ĺ����IJ����п���ʹ�ⶨ���ƫ�͵���____(����ĸ)��
a.�ζ���δ��ϴ��ֱ��ע����������Ʊ�Һ
b.�ζ��ܶ�ȡ��Һ���ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
c.��ƿ������ˮϴ����û�и���
���𰸡�CH3OCH3-12e-+3H2O=2CO2+12H+ 2H2O��4e��=4H++O2����4OH-��4e- =O2��+2H2O a NH4Cl��2HCl![]() 3H2����NCl3 Cl-��OH- 2ClO2��10I����8H����2Cl����5I2��4H2O 0.0270g����0.027g�� b
3H2����NCl3 Cl-��OH- 2ClO2��10I����8H����2Cl����5I2��4H2O 0.0270g����0.027g�� b
��������
��1�������϶�����ʧȥ�������ɶ�����̼�������������Һ��
��2����ͼ��֪�����Ϊ����������������ʧȥ���ӣ��������������ƶ���
��3�����������̿�֪�Ȼ����������Һ�н��е�⣬����������������������NCl3����ⷽ��ʽΪNH4Cl��2HCl![]() 3H2����NCl3����NCl3��Һ�м���NaClO2��������ClO2��NH3��X��X�к�Cl-��OH-��
3H2����NCl3����NCl3��Һ�м���NaClO2��������ClO2��NH3��X��X�к�Cl-��OH-��
��4����ClO2��⻯�ط���������ԭ�ֱ�������������ⵥ�ʣ�����������ԭ��Ӧ�Ĺ�����ƽ���ɣ�
�ڵ����������������2ClO2+10I-+8H+�T2Cl-+5I2+4H2O��I2+2S2O32-�T2I-+S4O62-�����2ClO2��5I2��10Na2S2O3���㣻
�����ı�Һ�����ƫСʱ���ⶨ���ƫ�͡�
(1)�õ���У����������ѷ���ʧ���ӵ�������Ӧ���为����ӦʽΪCH3OCH3-12e-+3H2O=2CO2+12H+��
�ʴ�Ϊ��CH3OCH3-12e-+3H2O=2CO2+12H+��
(2)��������Ϊϡ������Һ���������Һ�����ԣ�������������������Ӧ������ӦΪH2O�ŵ磬����O2��H+����缫��ӦΪ��2H2O��4e��=4H++O2����4OH-��4e- =O2��+2H2O����Һ�зָ������ҵ�ĤΪ�����ӽ���Ĥ���������������ƶ���H+ͨ�������ӽ���Ĥ����a�ң���a�ҵ�SO32-������Ӧ��H++SO32-=HSO3-����˵���a�ҵ�NaHSO3Ũ�����ӣ�NaHSO3��������Һ���ᾧ��ˮ�Ƶ�Na2S2O5���ù��̵Ļ�ѧ����ʽ2NaHSO3=Na2S2O5+H2O��
�ʴ�Ϊ��2H2O��4e��=4H++O2����4OH-��4e- =O2��+2H2O��a��
(3)�����������������������ԭ��Ӧ���ɿ�֪�����ʱ������Ӧ�Ļ�ѧ����ʽΪNH4Cl��2HCl![]() 3H2����NCl3��NCl3��NaClO2��Һ��Ӧ��NCl3+6NaClO2+3H2O=6ClO2��+NH3��+3NaCl+3NaOH��������ҺX�д������ڵ���������Cl-��OH-��
3H2����NCl3��NCl3��NaClO2��Һ��Ӧ��NCl3+6NaClO2+3H2O=6ClO2��+NH3��+3NaCl+3NaOH��������ҺX�д������ڵ���������Cl-��OH-��
�ʴ�Ϊ��NH4Cl��2HCl![]() 3H2����NCl3�� Cl-��OH-��
3H2����NCl3�� Cl-��OH-��
(4)��ClO2��⻯�ط���������ԭ�ֱ�������������ⵥ�ʣ���ϵ���ת�����غ�͵���غ㡢ԭ���غ��֪���÷�Ӧ�����ӷ���ʽΪ��2ClO2+10I-+8H+�T2Cl-+5I2+4H2O��
�ڵζ����յ����������Һ����ɫ��Ϊ��ɫ���Ұ��������Һ��ɫ���ٸı䣬
����Na2S2O3���ʵ���Ϊ0.02L��0.1mol/L=0.002mol�������2ClO2��5I2��10Na2S2O3��n(ClO2)=![]() n(Na2S2O3)����n(ClO2)=0.0004 mol������m(ClO2)=0.004mol��67.5g/mol=0.0270g����0.027g����
n(Na2S2O3)����n(ClO2)=0.0004 mol������m(ClO2)=0.004mol��67.5g/mol=0.0270g����0.027g����
��a. �ζ���δ��ϴ��ֱ��ע����������Ʊ�Һ�����ı�Һ�����ƫ�ⶨ���ƫ�ߣ��ʲ�ѡ��
b. �ζ��ܶ�ȡ��Һ���ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��������ı�Һ�����ƫСʱ���ⶨ���ƫ�ͣ���ѡ��
c. ��ƿ������ˮϴ����û�и����ʵ����Ӱ�죬�ʲ�ѡ��
�ʴ�Ϊ��b��

����Ŀ��ijС�����������װ�ý���ʵ�飬�ڡ�������Һ���������������������±���
ʵ�� | ���� | ���� |
�� | ��ʢ��Na2S��Һ�Ģ��г���ͨ��CO2������ | ���в�����ɫ��������Һ��pH���ͣ� ���в�����ɫ���ǣ��û�������ð���� |
�� | ��ʢ��NaHCO3��Һ�Ģ��г���ͨ��H2S���������� | ����ͬʵ��� |
���ϣ�CaS��ˮ��ȫˮ��
������ʵ��ó��Ľ�������ȷ����
A.���а�ɫ������CaCO3
B.������ҺpH���͵�ԭ���ǣ�H2S+Cu2+ == CuS��+2H+
C.ʵ�����з����ķ�Ӧ�ǣ�CO2+H2O+ S2== CO32+ H2S
D.��ʵ���͢��ܱȽ�H2CO3��H2S���Ե�ǿ��