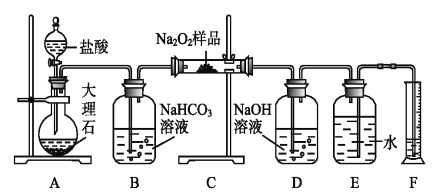
��Ŀ����
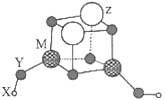
����Ŀ��±�ػ�������ICl��ICl3�Ⱦ�����±�ص������Ƶ����ʡ����÷�ӦI2+Cl2=2ICl��ʵ���ҿ�����ͼ��ʾװ�ã��г���������ȥ����ȡ����ICl3����֪��ICl���۵�Ϊ27.2�棬�е�Ϊ97.40C������ˮ�⣬�ܷ�����Ӧ��ICI+C12=IC13����������������ǣ� ��
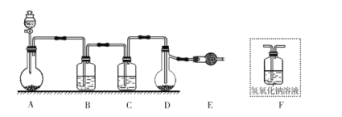
A.Բ����ƿ�еĹ������ΪKMnO4��KClO3
B.װ��B��C�е��Լ��ֱ�Ϊ����ʳ��ˮ��Ũ����
C.װ��E������Ϊ����β��������װ��F���
D.����ĵμ��ٶȹ��죬ICl�IJ��ʻ����Խ���
���𰸡�C
��������
A.Aװ��Ϊ�����Ʊ�װ�ã���װ��Ϊ��Һ������װ�ã��ʿ���ʹ��KMnO4��KClO3��Ũ���ᷴӦ��ȡ������A��ȷ��
B.��ȡ�������к��������Ȼ����ˮ����װ��B��ʢ�б���ʳ��ˮ��ȥHCl��װ��C��ʢ��Ũ�����ȥH2O��B��ȷ��
C.��ΪICl����ˮ�⡢�����ж���װ��E������Ϊ����β���ͷ�ֹ������ˮ��������Dװ�ã�װ��F�е���������������β�������ܷ�ֹˮ��������Dװ�ã�C����
D.������ĵ��ٹ����ʹ��Ӧ���ھ��ң�������ICI������Ӧ����IC13��ʹICl�IJ��ʽ��ͣ�D��ȷ��
��ѡC��

 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д�����Ŀ�����ڷ��õ�FeSO4��Һ�ױ����������ʣ�ij��ȤС�����������ʵ�飺
(1)���ʵ�����FeSO4��Һ�ı��ʳ̶�
ʵ�鷽�� | ʵ������ | ʵ����� | |
����1 | ȡ�����Һ���Թ��У������еμ�KSCN��Һ | ________ | FeSO4��Һ���ֱ��� |
����2 | _____ | _________ | |
�� ������������������
�� ��Ҫʹ���ֱ��ʵ�FeSO4��ԭ��������__________��(д���ӷ�Ӧ����ʽ)
(2)�������ֱ�����FeSO4��Һ�Ʊ�Fe2O3
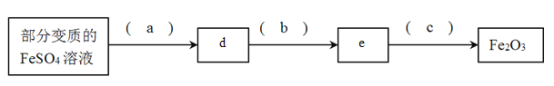
����д���и��գ�a._____b._______c.________d._____e.______
����100mL�ñ�����Һ�Ƶ�1.6gFe2O3�������ǰFeSO4��Һ��Ũ��Ϊ__________��
(3)FeSO4��������������ʹ��ʱ������ά����Cͬ����ͬѧ�ײ²�ά����C�ɽ�Fe3+ת��ΪFe2+���������������ա�Ϊ����֤��һ���룬���������ʵ�飺
ʵ�鷽�� | ʵ������ |
ȡ���� Fe2(SO4)3��Һ���Թ��У�����ά����CƬ�����ܽ�μ����Ը��������Һ�� | ��ɫ��ȥ |
������ʵ���ܷ�ó���ά����C�ɽ�Fe3+ת��ΪFe2+���Ľ��ۣ���˵������_______��
����Ŀ��ij�о���ѧϰС��Ϊ��̽������ĵ������������������ʵ��:
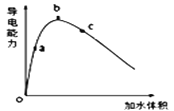
��1��ȡ����������250mL 0.4 mol��L��1�Ĵ�����Һ����0.4mol��L��1�Ĵ�����Һϡ�ͳ�����Ũ�ȵ���Һ������NaOH����Һ�����������Һ��Ũ�Ƚ��б궨���ش��������⣺
�ٽ�һ�������ı������ˮϡ�����У���Һ�ĵ��������仯��ͼ��ʾ����ϡ��������Һ��pH�ɴ�С��˳��____������ĸ����
��Ϊ�궨�ô�����Һ��ȷŨ�ȣ���0.2000mol��L-1��NaOH��Һ��20.00mL������Һ���еζ������εζ�����NaOH��Һ��������£�
ʵ����� | 1 | 2 | 3 | 4 |
����NaOH��Һ�����(mL) | 20.05 | 20.00 | 18.80 | 19.95 |
�ô�����Һ��ȷŨ��Ϊ_____________������С�������λ���������궨�����У���ɲⶨ���ƫ�ߵ�ԭ�������_____________����ѡ����ѡ���÷֣���
a��δ�ñ�Һ��ϴ��ʽ�ζ���
b���ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�������������ȷ
c��ʢװδ֪Һ����ƿ������ˮϴ����δ�ô���Һ��ϴ
d���ζ����յ����ʱ���ֵζ��ܼ��촦����һ����Һ
��2����С��ͬѧ̽��Ũ�ȶԴ������̶ȵ�Ӱ��ʱ����pH�Ʋⶨ25��ʱ��ͬŨ�ȵĴ����pH���������£�
����Ũ��( mol��L-1) | 0.0010 | 0.0100 | 0.0200 | 0.1000 | 0.2000 |
pH | 3.88 | 3.38 | 3.23 | 2.88 | 2.73 |
�ٸ��ݱ������ݣ����Եó�������������ʵĽ��ۣ�����Ϊ�ó��˽��۵�������___________��
�ڼ�����pH��ֽ�� 0.1mol��L-1 ������ҺpH�ķ���___________��