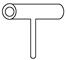
��Ŀ����
ijʵ��С��ͬѧΪ��̽��ͭ��Ũ����ķ�Ӧ������������ʵ�飬ʵ��װ����ͼ��ʾ��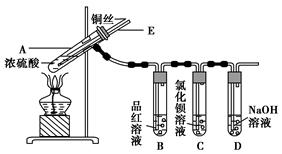
ʵ�鲽�裺
����������ͼ��ʾ��װ�ã����������ԣ��ټ����Լ���
�ڼ���A�Թܣ���B�Թ���Ʒ����Һ��ɫ��Ϩ��ƾ��ƣ�
�۽�Cu˿���ϳ鶯�뿪Һ�档
��ش��������⣺
(1)A�Թ��з�����Ӧ�Ļ�ѧ����ʽΪ ��
(2)�ܹ�֤��ͭ��Ũ���ᷴӦ���������ʵ�������� ��
(3)��ʢ��BaCl2��Һ��C�Թ��У����˵��ܿ��������⣬���������������������е���Һ�ֳ����ݣ��ֱ�μ�������Һ�������������Ļ�ѧʽ������ж�Ӧ��λ�á�
| �μӵ���Һ | ��ˮ | ��ˮ |
| �����Ļ�ѧʽ | | |
д������SO2���ֻ�ԭ�Ե����ӷ�Ӧ����ʽ�� ��
(4)ʵ����Ϻ���Ϩ��ƾ��ƣ����ڵ���E�Ĵ��ڣ��Թ�B�е�Һ�岻�ᵹ�����Թ�A�У���ԭ���� ��
(5)ʵ����Ϻ�װ���в����������ж������ܴ����ϵĽ�����Ϊ�˷�ֹ�����������������Ⱦ���������װ��ǰ��Ӧ����ȡ�IJ����� ��
(6)��SO2����ͨ�뺬��n mol Na2S����Һ�У���ַ�Ӧ����Һ�г��ֻ�ɫ���ǣ��Է�������Һ���������SO2���� mol(�������ܽ��SO2)��
(1)Cu��2H2SO4(Ũ)  CuSO4��SO2����2H2O
CuSO4��SO2����2H2O
(2)B�Թ���Ʒ����Һ��ɫ
(3)BaSO4��BaSO3��SO2��Cl2��2H2O=4H����SO42-��2Cl��(��Ba2����SO2��Cl2��2H2O=BaSO4����4H����2Cl��)
(4)��A�Թ�������ѹǿ��Сʱ��������E���ܽ���A�Թ��У�ά��A�Թ���ѹǿƽ��
(5)��E���ܿ���A�Թ��л����ع��������Ŀ�������������SO2�������NaOH��Һ�У�ʹ֮����ȫ����
(6)2.5n
����

��֪��ICl���۵�Ϊ13��90C���е�Ϊ97��40C����ˮ�⣬���ܷ�����Ӧ��ICl(l��+ Cl2(g��=ICl3(l)
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽ��____________ ��
��2��װ��B��������______��������װ��F����װ��E������____________ ��
��3�����Ƶõ�ICl����������ICl3���ʣ��ᴿ�ķ�����______ (���ţ���
| A������ | B�������ᾧ | C������ | D����Һ |
��

��ICl+KI��I2+KCl
��I2��2Na2S2O3��2NaI+Na2S4O6
ʵ��1:��5��00g����֬��Ʒ�������Ȼ�̼���γ�100mL��Һ������ȡ��ʮ��֮һ������20mLijICl�ı�������Һ(����������ַ�Ӧ��������KI��Һ�����ɵĵⵥ����a mol��L-1��Na2S2O3����Һ�ζ�����ƽ��ʵ�飬������ĵ�Na2S2O3��Һ��ƽ�����ΪV1mL��
ʵ��2(�հ�ʵ�飩��������֬��Ʒ�������������衢�����Լ���������ʵ��1��ȫ��ͬ��������ĵ�Na2S2O3��Һ��ƽ�����ΪV2mL��
�ٵζ������п���______ ��ָʾ����
�ڵζ���������Ҫ����������ᵼ��V1______(�ƫ��ƫС����
��5��00g����֬��Ʒ�����ĵ�ICl�����ʵ���Ϊ__mol���ɴ����ݾ����㼴����ø���֬�IJ����Ͷȡ�
��1��ij��ȤС����ʵ����̽����ҵ�ϳ�����Ļ�ѧԭ����
�ٰ��Ĵ�������ͼa��̽�����Ĵ������ļ���װ�ã�ʵ���й۲쵽��ƿ�в�˿���ֺ��ȣ��к���ɫ�������ɻ���̲��������̵ijɷ��� ���ѧʽ����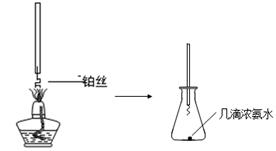
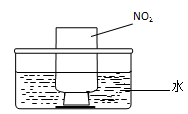
ͼa ͼb
��NO2�����գ���ͼb��ʾ����һƿNO2������ˮ���У���ˮ���ƿ�����Ƭ���ɹ۲쵽�������� ��
��2������������Һ��Fe3+ˮ������ػ�ɫ������Fe(NO3)3��ҺΪ�������ʵ��̽��Ӱ������ˮ��̶ȵ����ء�
��д��Fe(NO3)3ˮ������ӷ���ʽ ��
�ڲ���ʾ������±�ʵ�鷽������ơ�
��ѡ���ϣ�0.05mol?L-1Fe(NO3)3��0.5mol?L-1Fe(NO3)3��1.0mol?L-1HNO3��1.0mol?L-1NaOH��NaHCO3���塢����ˮ����ˮ����pH�Ƽ���������������
| ����Ӱ������ | ʵ����� | Ԥ������ͽ��� |
| ��Һ������� | ȡ����0.5mol?L-1Fe(NO3)3���Թ��У����뼸��1mol?L-1HNO3�� | �ػ�ɫ��Һ��ɫ��dz��˵����Һ������ǿ������Fe(NO3)3��ˮ�⡣ |
| �ε�Ũ�� | | |
| | | |
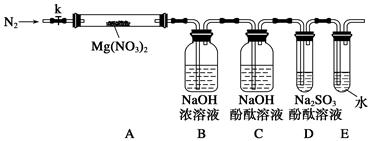
��ͼ���о�ͭ��Ũ����ķ�Ӧװ�ã�

��1��A�Թ��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2����Ӧһ��ʱ��ɹ۲쵽B�Թ��е�����Ϊ ��
��3��C�Թܿڽ���NaOH��Һ������������ ��
��4���罫B�Թܻ���D�Թܣ�����ֱ����������BaCl2��Һ��ͨ����һ�����壬������ɫ����������������� �� ����Ҫ����һ�ֻ������һ�ֵ��ʵĻ�ѧʽ��������Ҫ���ɼ�װ������װ�á���
��5��ʵ�������֤��A�Թ��з�Ӧ���ò����Ƿ���ͭ���ӵIJ��������� ��
��6����ͭ��Ũ���ᷴӦ�Ĺ����У������к�ɫ���ʳ��֣�������������������ϡ�
����
| ����1 |  ����ͭ��Ũ���ᷴӦ������ɫ���ʵ�������� |
| ����2 | X���߾������������ͭ��Ũ���ᷴӦ���ɵĺ�ɫ����ΪCu2S��CuS��Cu7S4�е�һ�ֻ��֡� |
�������Ͽɵó�����ȷ������ ��
a��ͭ��Ũ���ᷴӦʱ���漰�ķ�Ӧ���ܲ�ֹһ��
b������Ũ��ѡ���ʵ����ɱ����������г��ֺ�ɫ����
c���÷�Ӧ����������֮һ������Ũ�ȡ�15 mol��L
d������Ũ��Խ��ɫ����Խ����֡�Խ����ʧ
ij��ѧ̽��С������SO2�Ļ�ѧ���ʽ�������̽��,������������ʵ�鱨�档
| ���� | ��� | ��ѧ�� ��Ԥ�� | ʵ����֤ | ||
| ʵ����� | ʵ������ | ʵ��(������ ����ʽ��ʾ) | |||
| ���� ���� | ���� ������ | ��ˮ ��Ӧ | ��ʢ��SO2������Թܵ�����ˮ��,���ⶨ�Թ�����Һ��pH | �� | SO2+H2O H2SO3 H2SO3 |
| ��� ��Ӧ | �� | ���ְ� ɫ���� | �� | ||
(2)��̽��С�黹����SO2��SԪ�صĻ��ϼ�,Ԥ�Ⲣͨ��ʵ��̽��SO2���������ʡ�̽��������ѡ�õ�ʵ��ҩƷ��:Ũ���ᡢ�������ƹ��塢Na2S��Һ�����Ը��������Һ��Ʒ����Һ�ȡ�̽�����̵�ʵ��װ��ͼ��ͼ��ʾ,��ش��������⡣
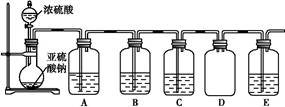
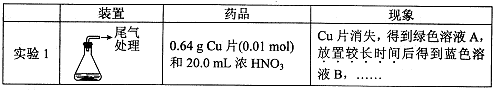
��������������
| װ�� | ҩƷ | ���� |
| A | | ��֤��������Ļ�ԭ�� |
| B | | |
| C | Ʒ����Һ | |
��A�з�����Ӧ�����ӷ���ʽΪ ��
��ʵ��ʱC�е�ʵ�������� ��
��Dװ�õ������� ��E��β������װ��,������ΪEװ���п��Լ���������Ba(NO3)2��Һ,����Ϊ�Ƿ����,�������ӷ���ʽ����˵��: ��

 Cu��NO2��42-����ɫ��
Cu��NO2��42-����ɫ��