��Ŀ����
����Ŀ��ijУ��ѧ��ȤС���ͬѧ�õζ�����һ��������Na2SO4��NaOH��Ʒ��NaOH�ĺ������вⶨ���ش��������⣺
(1)�÷�����ƽȷ��ȡ����Ʒ5.0 g��ȫ������ˮ���Ƴ�1 000.0 mL����Һ����____(����������)��ȡ20.00 mL������ƿ�У��μӼ��η�̪�����⡣���к͵ζ��еζ��յ��������___��
(2)��0.10 mol��L-1ϡ����ζ�δ֪Ũ�ȵ�NaOH��Һ��ʵ���������±���ʾ��
ʵ���� | ����NaOH��Һ�����/mL | ϡ��������/mL |
1 | 20.00 | 24.01 |
2 | 20.00 | 23.99 |
3 | 20.00 | 22.10 |
������Ʒ��NaOH����������Ϊ______��
(3)���ζ�ǰ���ζ��ܼ�������ݣ��ζ�����������ʧ����ʹ������____ (����ƫ��������ƫ������������������ͬ)�����ζ������в�������ƿ����Һ��������ʹ������_____��������ʽ�ζ��ܶ���ʱ���ζ�ǰ���Ӷ������ζ�����ȷ��������������____��
���𰸡���ʽ�ζ��� ��Һ�ɺ�ɫ��Ϊ��ɫ����30 s�ڲ���ɫ 96% ƫ�ߡ� ƫ�� ƫ��
��������
��1����������Na2SO4��NaOH��Ʒ������ˮ��Һ�Լ��ԣ��ݴ�ѡ��������ѡȡ��̪��ָʾ�������ݷ�Ӧ�յ���Һ��ɫ������
��2�������βⶨ��ֵ���ϴ���ȥ����������������ֵ����ƽ��ֵ����Ϸ�Ӧ������ϵ�����������Ƶ�����������
��3���Լ��������������ĵı�Һ����Ķ��������������c(��) =  �����жϡ�
�����жϡ�
(1)��������Na2SO4��NaOH��Ʒ������ˮ��Һ�Լ��ԣ��ü�ʽ�ζ���ȡ�ã�����ζ��ⶨ�����������ʵ���ǰ��Ӧ��Һ�����ԣ���̪pH��ɫ��ΧΪ��810���ζ��յ�ʱ����Һ�ɺ�ɫ��Ϊ��ɫ����30s�ڲ���ɫ��
�ʴ�Ϊ����ʽ�ζ��ܣ���Һ�ɺ�ɫ��Ϊ��ɫ����30s�ڲ���ɫ��
(2)�����βⶨ��ֵ���ϴ���ȥ����������������ֵ����ƽ��ֵ=![]() =24mL����Ϸ�Ӧ������ϵ�����������Ƶ����ʵ���=
=24mL����Ϸ�Ӧ������ϵ�����������Ƶ����ʵ���=![]() =0.12mol��������Ʒ�У�NaOH�������ٷֺ���=
=0.12mol��������Ʒ�У�NaOH�������ٷֺ���=![]() ��100%=96%��
��100%=96%��
�ʴ�Ϊ��96%��
(3)���ζ�ǰ���ζ��ܼ�������ݣ��ζ�����������ʧ�������Һ����Ķ���ƫ��������Ľ��ƫ�ߣ�
���ζ������в�������ƿ����Һ�����������ĵı�Һ��������С����ʹ������ƫ�ͣ�
������ʽ�ζ��ܶ���ʱ���ζ�ǰ���Ӷ�����ȡ��ֵ���ζ�����ȷ���������������ı���Һ�����С������������ƫ�ͣ�
�ʴ�Ϊ��ƫ�ߣ�ƫ�ͣ�ƫ�͡�

����Ŀ��������һ�������Դ������CO��ˮ������Ӧ�Ʊ����������仯����ͼ��ʾ��

(1)�÷�ӦΪ���淴Ӧ����800 ��ʱ����CO����ʼŨ��Ϊ2.0 mol��L-1��ˮ��������ʼŨ��Ϊ3.0 mol��L-1���ﵽ��ѧƽ��״̬���CO2��Ũ��Ϊ1.2 mol��L-1����˷�Ӧ��ƽ�ⳣ��Ϊ___�������¶����߸÷�Ӧ�Ļ�ѧƽ�ⳣ���ı仯������___�������������������С��������������
(2)ij�¶��£��÷�Ӧ��ƽ�ⳣ��ΪK=1/9�����¶����ڼס��ҡ������������ܱ�������Ͷ��H2O(g)��CO(g)������ʼŨ�����±���ʾ�������жϲ���ȷ����____(����ĸ)��
��ʼŨ�� | �� | �� | �� |
c(H2O)/mol��L-1 | 0.010 | 0.020 | 0.020 |
c(CO)/mol��L-1 | 0.010 | 0.010 | 0.020 |
A. ��Ӧ��ʼʱ�����еķ�Ӧ������죬���еķ�Ӧ��������
B. ƽ��ʱ�����кͱ���H2O��ת���ʾ���25%
C. ƽ��ʱ������c(CO2)�Ǽ��е�2������0.015 mol��L-1
D. ƽ��ʱ������H2O��ת���ʴ���25%
(3)һ�������£����淴ӦN2(g)+3H2(g)![]() 2NH3(g)����H<0���ﵽƽ���
2NH3(g)����H<0���ﵽƽ���
�ټӴ�����v(��)��v(��)�������仯���ұ仯�ı���__(��������������������)��
����С���ʹ��ϵѹǿ������v(��)___��v(��)___(����������������С������������)��v(��)�仯�ı���__v(��)�仯�ı���(��������������С��������������)��
�۽��£�v(��) ____��v(��)__(����������������С������������)��
���º�ѹ����ͨ��һ������He���ﵽ��ƽ��ʱ��N2��ת����__��c(H2)��__(����������������С������������)��
����Ŀ���Լ����CO2�ĸ�Ч���ò����ܻ��������ů�����Ҷ�����ݽߵ�ʯ����ԴҲ��һ���IJ������ã������������CO2������Ӧ�У�
��Ӧ��i����2CH4(g)��O2(g)![]() 2CO(g)��4H2(g)�� ��H=��71.4kJmol-1
2CO(g)��4H2(g)�� ��H=��71.4kJmol-1
��Ӧ��ii����CH4(g)��CO2(g)![]() 2CO(g)��2H2(g) ��H=+247.0 kJmol-1
2CO(g)��2H2(g) ��H=+247.0 kJmol-1
��1��д����ʾCOȼ���ȵ��Ȼ�ѧ����ʽ��_______________________________��
��2�������������Ϊ2L�ĺ����ܱ������У���ʼʱ��������Ӧ�����������ʣ�����ͬ�¶��½��з�Ӧ��ii����CH4(g)��CO2(g)![]() 2CO(g)��2H2(g) ��������������Ӧ����CO2��ƽ��ת�������±���ʾ��
2CO(g)��2H2(g) ��������������Ӧ����CO2��ƽ��ת�������±���ʾ��
���� | ��ʼ���ʵ���(n) / mol | CO2�� ƽ��ת���� | |||
CH4 | CO2 | CO | H2 | ||
�� | 0.1 | 0.1 | 0 | 0 | 50�� |
�� | 0.1 | 0.1 | 0.2 | 0.2 | / |
��������˵����Ӧ�ﵽƽ��״̬��_________��
A��v��(CH4) =2v��(CO)
B�������ڸ����ʵ�Ũ������c(CH4)��c(CO2)=c2(CO)��c2(H2)
C�������ڻ���������ѹǿ���ٱ仯
D�������ڻ�������ܶȱ��ֲ���
�����������ڷ�Ӧ�ӿ�ʼ��ƽ�����õ�ʱ��Ϊt min����t min�ڸ÷�Ӧ��ƽ����Ӧ����Ϊ��v(H2) = ________���ú�t�ı���ʽ��ʾ����
���ﵽƽ��ʱ��������������CO�����ʵ����Ĺ�ϵ���㣺2n(CO)��_______n(CO)������������������������������
��3����CH4(g)��O2(g)�����ʵ�����Ϊ4:3����ʢ�д����ĺ����ܱ������ڣ�����������Ӧ��i����2CH4(g)��O2(g)![]() 2CO(g)��4H2(g)����ͬʱ����ڲ��CO���������[��(CO)]���¶ȣ�T���Ĺ�ϵ��ͼ��ʾ��
2CO(g)��4H2(g)����ͬʱ����ڲ��CO���������[��(CO)]���¶ȣ�T���Ĺ�ϵ��ͼ��ʾ��
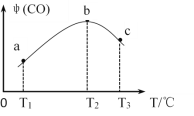
�� T2��ʱ��CO�����������ԭ����_____________��
����T2��ʱ����������ʼѹǿΪP0��ƽ��ʱCO���������Ϊ20������Ӧ��ƽ�ⳣ��KP =_______����ƽ���ѹǿ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ�����������
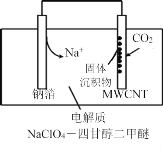
��4��2016���ҹ�������Ա���ݷ�ӦNa+CO2�� Na2CO3+C��δ��ƽ�� ���Ƴ�һ���������ɺ�����Na-CO2��ء��ŵ�ʱ�õ����������CO2�����ʱ��������CO2����ŵ�ʱ�Ĺ���ԭ����ͼ��ʾ����֪���յ�ȫ��CO2�У���![]() ת��ΪNa2CO3��������ڶ��̼���ܣ�MWCNT���缫���棬д���ŵ�ʱ�����ĵ缫��Ӧʽ��_________________��
ת��ΪNa2CO3��������ڶ��̼���ܣ�MWCNT���缫���棬д���ŵ�ʱ�����ĵ缫��Ӧʽ��_________________��
����Ŀ��ȼú�������к��� SO2��Ϊ�����������������������ö��ַ���ʵ����������
��.(1)��ʪʽ���շ����������ռ��� SO2 ������Ӧ�Ӷ����������Լ����ʺ������÷����ռ�����_____(����ĸ���)��
a. ʯ���� b.CaCl2��Һ
(2)ij�������ú� SO2 ������������Cr2O72-�����Է�ˮ���������з�Ӧ��ĸ�Ԫ����Cr3+��ʽ���ڣ������������£�
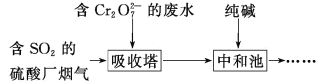
���� SO2 ����������ˮʱ�������� SO2 ��_____�ԡ�
���������з�����Ӧ�����ӷ���ʽΪ_____��
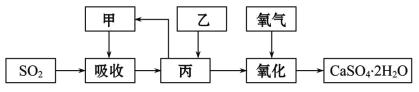
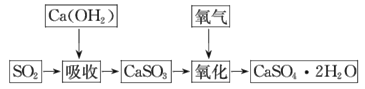
��.ʯ��-ʯ�෨���ռ�dz��õ���������ʯ��-ʯ�෨�����շ�ӦΪCa(OH)2+SO2= CaSO3��+H2O�����ղ�����������ɹܵ���������������������ӦΪ2CaSO3+O2+4H2O =2CaSO4��2H2O����������ͼ��
�ռ�����շ�ӦΪ2NaOH+SO2=Na2SO3+H2O���÷����ص����������Ƽ���ǿ�����տ졢Ч�ʸߡ���������ͼ��

��֪��
�Լ� | Ca(OH)2 | NaOH |
�۸�(Ԫ/kg) | 0.36 | 2.9 |
���� SO2 �ijɱ�(Ԫ/mol) | 0.027 | 0.232 |
(3)ʯ��-ʯ�෨���ռ��ȣ�ʯ��-ʯ�෨���ŵ���_______��ȱ����_______��
(4)ijѧϰС����ʯ��-ʯ�෨���ռ�Ļ����ϣ����һ���Ľ��ġ���ʵ������ѭ������������������ͼ�еļס��ҡ�������_____��_____��_____(�ѧʽ)