��Ŀ����
����Ŀ��������ѧ��ѧ֪ʶ������⣺
![]() �ڸ�����CuO�ֽܷ�����
�ڸ�����CuO�ֽܷ�����![]() ���Դ�ԭ�ӽṹ�ǶȽ�����ԭ�� ______
���Դ�ԭ�ӽṹ�ǶȽ�����ԭ�� ______ ![]() ����Ԫ��ԭ�ӵ���Χ�����Ų��������ɽ����ڱ��ֳ��������Ԫ��Cu���� ______ ��
����Ԫ��ԭ�ӵ���Χ�����Ų��������ɽ����ڱ��ֳ��������Ԫ��Cu���� ______ ��![]()
![]() ����
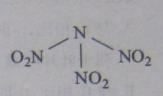
����![]() ��һ����״���ӣ�����������
��һ����״���ӣ�����������![]() ��Ϊͬ���칹�壬������ڸ�ԭ���������Ѵﵽ�ȶ��ṹ����д������Ľṹʽ ______
��Ϊͬ���칹�壬������ڸ�ԭ���������Ѵﵽ�ȶ��ṹ����д������Ľṹʽ ______ ![]() ���е�C���ӻ�����Ϊ ______ ��
���е�C���ӻ�����Ϊ ______ ��
![]() ԭ�ӻ�������Χ�н϶���������Ŀչ������һЩ���ӻ������γ������
ԭ�ӻ�������Χ�н϶���������Ŀչ������һЩ���ӻ������γ������![]() ��Feԭ�ӻ������γ������ķ��ӻ�����Ӧ�߱��Ľṹ������ ______ ��
��Feԭ�ӻ������γ������ķ��ӻ�����Ӧ�߱��Ľṹ������ ______ ��
![]() ������������һ�����͵ij�Ӳ����ĥ�����µĽṹ���ϣ��侧���ṹ����ʯ���ƣ�һ���þ����к��� ______ ����ԭ�ӣ� ______ ����ԭ�ӣ��赪ԭ�Ӱ뾶Ϊapm�����ԭ�Ӱ뾶bpm����þ����Ŀռ������� ______
������������һ�����͵ij�Ӳ����ĥ�����µĽṹ���ϣ��侧���ṹ����ʯ���ƣ�һ���þ����к��� ______ ����ԭ�ӣ� ______ ����ԭ�ӣ��赪ԭ�Ӱ뾶Ϊapm�����ԭ�Ӱ뾶bpm����þ����Ŀռ������� ______ ![]() �ú�a��b�Ĵ���ʽ��ʾ
�ú�a��b�Ĵ���ʽ��ʾ![]()

���𰸡� �ṹ��![]() Ϊ
Ϊ![]() ����
����![]() Ϊ
Ϊ![]() ȫ�������ȶ� ds
ȫ�������ȶ� ds ![]() sp�ӻ� ���йµ��Ӷ�
sp�ӻ� ���йµ��Ӷ� ![]() 4
4 ![]()
����������1������Cu2+��Cu+�ļ۵����Ų�ʽ������Cu����ds����
��2�����ݼۼ�������������ڸ�ԭ���������ﵽ�ȶ��ṹ������ĽṹʽΪH��O��C![]() N������CΪsp�ӻ���
N������CΪsp�ӻ���
��3��������������������
��4��������̯����ȷ�������������������ɵ�ԭ�Ӱ뾶����ԭ�Ӱ뾶ȷ�������ı߳����ɾ����߳����㾧����������ɾ�����������ԭ�Ӱ뾶���㾧����ԭ�ӵ�����������㾧���Ŀռ������ʡ�
��1��CuO�к�Cu2+��O2-��Cu2O�к�Cu+��O2-��Cu2+�ļ۵����Ų�ʽΪ3d9��Cu+�ļ۵����Ų�ʽΪ3d10ȫ�������ȶ���������CuO�ֽܷ�����Cu2O����̬Cuԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s1��Cuλ�ڵ������ڵ�IB�壬Cu����ds����
��2�����ݼۼ�������������ڸ�ԭ���������ﵽ�ȶ��ṹ������ĽṹʽΪH��O��C![]() N������C�γ�2���Ҽ���C��û�йµ��Ӷԣ�Cԭ��Ϊsp�ӻ���
N������C�γ�2���Ҽ���C��û�йµ��Ӷԣ�Cԭ��Ϊsp�ӻ���
��3��Feԭ�ӻ����Ӿ��пչ��������Feԭ�ӻ������γ������ķ��ӻ�����Ӧ�߱��Ľṹ�����ǣ����йµ��Ӷԡ�
��4��������̯������1�������к�N��8![]() +6
+6![]() =4������B��4������ԭ�Ӱ뾶Ϊapm����ԭ�Ӱ뾶Ϊbpm�����ı߳�Ϊ
=4������B��4������ԭ�Ӱ뾶Ϊapm����ԭ�Ӱ뾶Ϊbpm�����ı߳�Ϊ![]() pm�����������Ϊ
pm�����������Ϊ![]() ��a+b��3pm3��������ԭ�ӵ����Ϊ4
��a+b��3pm3��������ԭ�ӵ����Ϊ4![]() ��
��![]() +
+![]() ��pm3�������Ŀռ�������Ϊ4
��pm3�������Ŀռ�������Ϊ4![]() ��
��![]() +
+![]() ��pm3
��pm3![]() [
[![]() ��a+b��3pm3]
��a+b��3pm3]![]() 100%=
100%=![]() 100%��
100%��

����Ŀ�����Ĺ̶�һֱ�ǿ�ѧ���о�����Ҫ���⣬�ϳɰ������˹��̵��Ƚϳ���ļ�������ԭ��ΪN2 (g)+3H2 (g)![]() 2NH3(g)��
2NH3(g)��
(1)��֪ÿ�ƻ�1mol�йػ�ѧ����Ҫ���������±���
H-H | N-H | N-N | N��N |
435.9KJ | 390.8KJ | 192.8KJ | 945.8KJ |
(1)��Ӧ���������_________(�>���� ��<��)���������������
(2)��һ���¶��¡���2L�ܱ������м���2 molN2��6 mol H2�����ʵ��Ĵ��������£�������Ӧ N2 (g)+3H2 (g)![]() 2NH3(g)��10min��ﵽƽ�⣬��ʱʣ��4.5mol H2��
2NH3(g)��10min��ﵽƽ�⣬��ʱʣ��4.5mol H2��
������������˵���˷�Ӧ�ﵽƽ��״̬����________________________��
a����������ѹǿ���� b��v(H2)����v(H2)���� c��N2��H2��Ũ�����
d�� 2 mol NH3���ɵ�ͬʱ��3 moH��H������ e��NH3��Ũ�Ȳ��ٸı�
��0��10 min�ڵ�ƽ����Ӧ����v(H2) ��______mol/(Lmin)��10��ĩNH3��Ũ����______mol/L��N2 �ĵ����ʵ���________mol
��ij�¶�ʱ����һ��2L���ܱ�������X��Y��Z�����������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ���ݴ˻ش�

(1)�÷�Ӧ�Ļ�ѧ����ʽΪ_______________________��
(2)�ӿ�ʼ��2min��Z��ƽ����Ӧ����Ϊ____________mol/(L��min)��
(3)�ı��������������Լӿ컯ѧ��Ӧ���ʵ���_________��
A�������¶� B����С����X�����ʵ��� C����Сѹǿ D����������Z�����ʵ��� E����С�ݻ�
F��ʹ��Ч�ʸ��ߵĴ���
(4)�÷�Ӧ����Ϊ���ȷ�Ӧ����������Ϊ��������(��������Ƚ���)����ƽ������ʱ�佫______��
a���ӳ� b������ c������ d����ȷ��