��Ŀ����
����Ŀ�����ֶ�����Ԫ��A��B��C��D��E��ԭ��������������Aԭ�Ӻ�����������A��Cͬ���壬A��B�γɵĻ�������ˮ�гʼ��ԣ�����D�ڵ���E�п�ȼ�ղ�����ɫ�������ش��������⣺
(1)��B�⣬����Ԫ�صĵ縺�ԴӴ�С����Ϊ ��(��Ԫ�ط��ű�ʾ)
(2)B���⻯����ͬ��������������������γɵ��⻯��е��ɸߵ��͵�����˳���� ���û�ѧʽ��ʾ),ԭ���� ��
(3)A��D�γɵĻ����������ԭ�ӵ��ӻ�����Ϊ ��A��B�γɵĻ�����BA5�д��ڵĻ�ѧ������Ϊ ��
(4)����D��һ�ֺ�����ɱ�ʾΪH3DO3�����Ƕ�Ԫ���ᣬ��д����������������������Һ��Ӧ�����ӷ���ʽ____________��
(5)C ��һ��������ľ����ṹ��ͼ��ʾ,��������a=0.566 nm��������Cԭ�ӵ���λ��Ϊ ���˾�����ܶ�(g��cm��3)Ϊ (ֻ��ʽ������)��

���𰸡�(1)Cl>P>H>Na
(2)�е㣺NH3��AsH3��PH3�� NH3���γɷ��Ӽ�������е���ߣ�AsH3��Է���������PH3���Ӽ�������������AsH3��PH3�е����
(3)sp3�����Ӽ������ۼ���(4)H3PO3+2OH-=HPO32-+2H2O
(5)4��![]() ��
��
��������
������������ֶ�����Ԫ��A��B��C��D��E��ԭ��������������Aԭ�Ӻ��������ӣ�AΪHԪ�أ���A��Cͬ���壬��CΪNaԪ�أ�A��B�γɵĻ�������ˮ�гʼ��ԣ�BΪNԪ�أ�����D�ڵ���E�п�ȼ�ղ�����ɫ��������DΪPԪ�أ�EΪClԪ�ء�
(1)Ԫ�صķǽ�����Խǿ���縺����ֵԽ��Ԫ�صĵ縺�ԴӴ�С����ΪCl>P>H>Na���ʴ�Ϊ��Cl>P>H>Na��
(2)NH3���γɷ��Ӽ�������е���ߣ�AsH3��Է���������PH3���Ӽ�������������AsH3��PH3�е�ߣ��ʴ�Ϊ���е㣺NH3��AsH3��PH3�� NH3���γɷ��Ӽ�������е���ߣ�AsH3��Է���������PH3���Ӽ�������������AsH3��PH3�е�ߣ�
(3)PH3��Pԭ�ӵļ۲���Ӷ�=3+![]() (5-1��3)=4����ȡsp3�ӻ���NH5����д��NH4H�����ڵĻ�ѧ�������Ӽ������ۼ����ʴ�Ϊ��sp3�����Ӽ������ۼ���
(5-1��3)=4����ȡsp3�ӻ���NH5����д��NH4H�����ڵĻ�ѧ�������Ӽ������ۼ����ʴ�Ϊ��sp3�����Ӽ������ۼ���
(4)H3PO3�Ƕ�Ԫ���ᣬ��������������������Һ��Ӧ�����ӷ���ʽΪH3PO3+2OH-=HPO32-+2H2O���ʴ�Ϊ��H3PO3+2OH-=HPO32-+2H2O��
(5)��������Ϊ���ӻ���������Ӱ뾶С�������Ӱ뾶������ͼʾ��������λ�ھ����Ķ�������ģ�������λ�ھ��������ģ���Na�ĸ���Ϊ8��O�ĸ���Ϊ8��![]() +6��
+6��![]() =4��N(Na)��N(O)=2��1�����γɵĻ�����ΪNa2O��������Oλ�ڶ��㣬Naλ�����ģ�ÿ����������1��Na��O�ľ��������ÿ������Ϊ8���������У�������ԭ�ӵ���λ��Ϊ8����ԭ�ӵ���λ��Ϊ4������������Ϊ
=4��N(Na)��N(O)=2��1�����γɵĻ�����ΪNa2O��������Oλ�ڶ��㣬Naλ�����ģ�ÿ����������1��Na��O�ľ��������ÿ������Ϊ8���������У�������ԭ�ӵ���λ��Ϊ8����ԭ�ӵ���λ��Ϊ4������������Ϊ![]() �����������Ϊ(0.566��10-7)cm3������F���ܶ�Ϊ
�����������Ϊ(0.566��10-7)cm3������F���ܶ�Ϊ![]() ���ʴ�Ϊ��4��
���ʴ�Ϊ��4��![]() ��
��

����Ŀ��

��1������������ʵ���Ũ����________mol/L��
��2��ij��ѧ��ȤС������������ʵ�ʵ��̽��ʱ����Ҫ490 mL 4.6 mol/L��ϡ���ᣬ����Ҫȡ________mL�ĸ����ᡣ
��3������ʱ������IJ�����������Ͳ���ձ����������ͽ�ͷ�ι�֮�⣬����Ҫ �����������ƣ���
��4��������Һ�����£�δ��˳�����У���a���ܽ⣬b��ҡ�ȣ�c��ϴ�ӣ�d����ȴ��e��������f������Һ��������ƿ��g�����ݵȲ���������ҡ�ȵ�ǰһ�������� ������д��ĸ��
��5�����������ƹ���ʾ��ͼ�У��д�����ǣ���д��ţ� ��
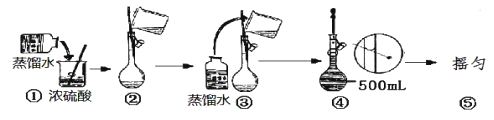
��6��������4.6 mol/L��ϡ����Ĺ����У��������������������Һ���ʵ���Ũ��ƫ�ߵ���
A��δ����ȴ���Ƚ���Һע������ƿ�� | B������ƿϴ�Ӻ�δ�����ﴦ�� |
C������ʱ���ӹ۲�Һ�� | D��δϴ���ձ��Ͳ����� |
����Ŀ���ֲ�����ѧ��ѧ����Ԫ��ԭ�ӽṹ�����������ʾ��
��� | Ԫ�� | �ṹ������ |
�� | A | A��һ�ֳ�������������һ���������Ǿ��д��Եĺ�ɫ���� |
�� | B | B��һ�ֳ���������ԭ�Ӻ������������Ӳ�����������Ϊż�� |
�� | C | C����̬�⻯����Һ����������� |
�� | D | DΪ�Һ�ɫ���н�������Ĺ��壬��̫���ܵ�ذ�ij��ò��� |
�� | E | Eԭ���������������ڲ����������3�� |
�� | F | F�����ڱ��п���������A�壬Ҳ�������������A�� |
(1)Aԭ�������ڱ��е�λ��Ϊ______________�����Ӱ뾶:B2+_________C3-(��"���ڡ�С�ڻ������)��
(2)����̬�⻯������ȶ��ԣ�D__________E(��"���ڡ�С�ڻ������)��
(3)��F��E�����γ�ԭ�Ӹ����ȷֱ�Ϊ2��1��1��1�����ֻ�����X��Y������X��Y��ˮ��Һ��ʵ�鷽����________________��
��F��C��ɵ����ֻ�����M��N�����ĵ������ֱ���X��Y��ȣ���M�ĵ���ʽΪ__________��N�ĽṹʽΪ___________��
(4)�û�ѧ����ʽ����C��E������Ԫ�صķǽ����Ե�ǿ��_________________��