��Ŀ����
����Ŀ��50ml0.50mol��L-1������50mL0.55mol��L-1NaOH��Һ������ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ�����зų��������ɼ����к��ȡ�
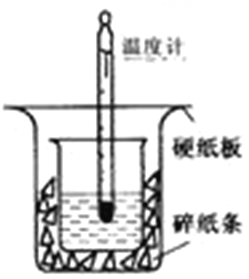
�ش��������⣺
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ���������_______��װ���л����ڵ�2�������� ��
��2����ʵ������У���ͬѧ��Ҫ�ⶨ����¼��ʵ�������� ������ţ���
A�����Ũ�� B������¶�
C����������Һ��Ũ�� D����������Һ���¶�
Eˮ�ı����� F��Ӧ������Һ����ֹ�¶�
��3��ʹ�ò�ȫ�������װ�ý���ʵ�飬ȡ50ml0.50mol��L-1������50mL0��55 mol��L NaOH��Һ��С�ձ��н����кͷ�Ӧ������ʵ���¶�ƽ������3��4�档��֪�кͺ����ɵ���Һ�� ������Ϊ4��18J����g���棩����Һ���ܶȾ�Ϊ1g��cm3��ͨ������ɵ��к��ȡ�H= ��
��4��ʵ���и���60mL0��50mol��L-1�����50mL0��55mol��L-1NaOH��Һ���з�Ӧ����������ȷ��ʵ�������ȣ����ų������� �����ȡ�����ȡ����������к��� �����ȡ�����ȡ�����
��5������ͬŨ�Ⱥ�����İ�ˮ����NaOH��Һ��������ʵ�飬��õ��к�����ֵ�� �����ƫ��ƫС������Ӱ�족����
���𰸡���1�����β��������������С�ձ�δ����ձ�������ƽ���ձ���δ������ֽ��
��2��BDF��3��-56.8kJ��mol-1��4������� ��ȣ�5��ƫС
��������
�����������1�������ȼƵĹ����֪��װ�õ�ȱ�������ǻ��β�����������к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹����������ձ�Ϊһ���ߣ���������ɢʧ�����2����������С�ձ�δ����ձ�������ƽ���ձ���δ������ֽ����
��2���ڸ�ʵ������У���ͬѧ��Ҫ�ⶨ��ʵ���������ᡢ��ij�ʼ�¶��Լ���Ӧ������Һ����ֹ�¶ȣ���Ϊ��BDF��
��3��50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ�кͷ�Ӧ����ˮ�����ʵ���Ϊ0.05L��0.50mol��0.025mol����Һ������Ϊ��100mL��1g/mL��100g���¶ȱ仯��ֵ��TΪ3.4����������0.025molˮ�ų�������ΪQ��mc��T��100g��4.18J/��g������3.4��=1421.2J������ʵ���õ��к�����H��-1.4212kJ/0.025mol����56.8kJ��mol-1��
��4����Ӧ�ų����������������Լ�������Ķ����йأ�����60mL 0.50mol/L�����50mL 0.55mol/L NaOH��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�ߣ������к��ȵľ���ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ��к�����ȣ�
��5����ˮΪ����������Ϊ���ȹ��̣������ð�ˮ����ϡ����������Һ��Ӧ����Ӧ�ų�������ƫС��