��Ŀ����
��15�֣���֪�������Լ�����±������þ�������ܼ��з�Ӧ�Ƶã������л��ϳ���Ӧ�ù㷺��
�ش��������⣺
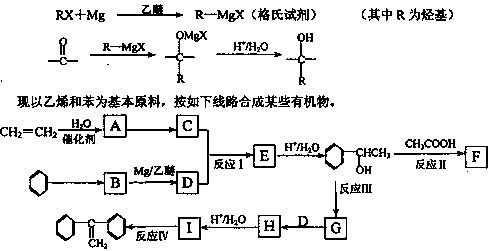
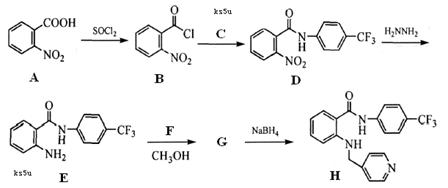
��1��C�Ľṹ��ʽ�ߣߣߣߣߣߣߣߣߣߡ�
��2����ӦI�������ǣߣߣߣߣߣߣߣߣߣߡ�
��3����ӦIII�������ǣߣߣߣߣߣߣߣߣߣߡ�
��4��д�����з�Ӧ�Ļ�ѧ����ʽ��Ҫ��ע����Ӧ��������
��Ӧ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
��Ӧ�����ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��5��G��ͬ���칹���У����б������ܷ���������Ӧ���л����Уߣߣߣߣ��֣���д������һ�ֵĽṹ��ʽΪ���ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�

�ش��������⣺
��1��C�Ľṹ��ʽ�ߣߣߣߣߣߣߣߣߣߡ�
��2����ӦI�������ǣߣߣߣߣߣߣߣߣߣߡ�
��3����ӦIII�������ǣߣߣߣߣߣߣߣߣߣߡ�
��4��д�����з�Ӧ�Ļ�ѧ����ʽ��Ҫ��ע����Ӧ��������
��Ӧ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
��Ӧ�����ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��5��G��ͬ���칹���У����б������ܷ���������Ӧ���л����Уߣߣߣߣ��֣���д������һ�ֵĽṹ��ʽΪ���ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��15�֣�
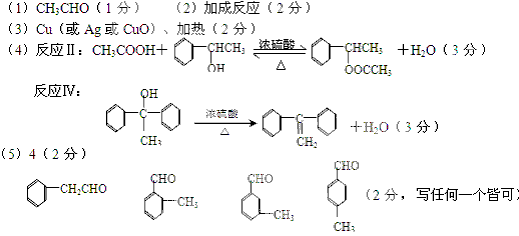

��

��ϰ��ϵ�д�
�����Ŀ
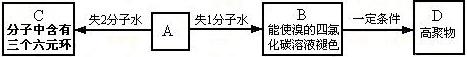

 ��R��R����Ϊ��H��CH3��NH2�ȡ�
��R��R����Ϊ��H��CH3��NH2�ȡ� ��һ�ֿ���������ҩ�������������Ϣ����
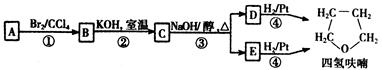
��һ�ֿ���������ҩ�������������Ϣ���� ��CH3COCl��CH3OHΪԭ�ϣ��ϳɹ��������Լ���ѡ���ϳ�·������ͼʾ�����£�
��CH3COCl��CH3OHΪԭ�ϣ��ϳɹ��������Լ���ѡ���ϳ�·������ͼʾ�����£�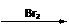 CH3CH2OH
CH3CH2OH 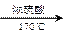 H2C��CH2 BrH2C��CH2Br
H2C��CH2 BrH2C��CH2Br
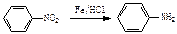
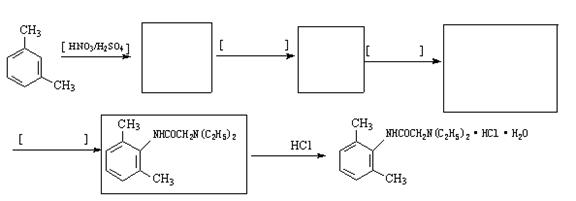

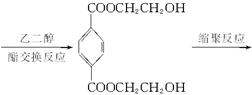
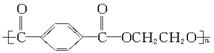 ?����ҵ����������ᴿ�����ý����ᴿ�ĶԱ���������������Ҷ���Ϊ��Ҫԭ�ϣ������й������̺ϳɵ�����֬��
?����ҵ����������ᴿ�����ý����ᴿ�ĶԱ���������������Ҷ���Ϊ��Ҫԭ�ϣ������й������̺ϳɵ�����֬��

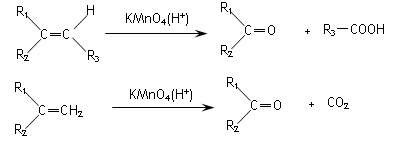
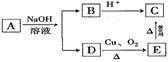
 C6H12O6+6O2 b��CO2 + 3H2
C6H12O6+6O2 b��CO2 + 3H2 CH3OH +H2O
CH3OH +H2O