��Ŀ����
���ž��õIJ��Ϸ�չ�����ǶԻ�������ҲԽ��Խ��ע��
(1)���ڿ�����Ⱦ����õ�Խ��Խ��Ĺ�ע������������Ҳ�������ڻӷ����л���Ⱦ�����
___________________��(����ĸ)
a���� b������ c������ d����ϩ
(2)���д�ʩ�У������ڽ���������Ⱦ��Ũ�ȵ���__________��(����ĸ)
a���ڿ���ͨ�� b���ճ�������������Ȼ�����ú��ȼ��
c��ʹ�á���ɫ������װ�β��� d�����ڷ�һЩ����̼
(3) ������ЧӦ����ȫ���ע�Ļ�������֮һ��CO2��Ŀǰ�����к�����ߵ�һ���������塣��ˣ����ƺ�����CO2�ǽ������ЧӦ����Ч;������CO2ת�����л������Чʵ��̼ѭ����CO2ת�����л�������Ӻܶ࣬�磺
a��6CO2 + 6H2O C6H12O6+6O2 b��CO2 + 3H2
C6H12O6+6O2 b��CO2 + 3H2 CH3OH +H2O
CH3OH +H2O
c��CO2 + CH4 CH3COOH d��2CO2 + 6H2
CH3COOH d��2CO2 + 6H2 CH2==CH2 + 4H2O
CH2==CH2 + 4H2O
���Ϸ�Ӧ�У�����ܵ��� ��ԭ�������ʣ�ԭ��������=������������������������������֮�ȣ���ߵ��� ��
(4) ������β����Ⱦ���ѱ������˹�ע�������飨C8H8���������͵ijɷ֣�Ҫʹ����������ȫȼ�գ����������������������O2ռ1/5�����������ȣ���ͬ�����£�Ϊ ��������λС������
(1)���ڿ�����Ⱦ����õ�Խ��Խ��Ĺ�ע������������Ҳ�������ڻӷ����л���Ⱦ�����
___________________��(����ĸ)
a���� b������ c������ d����ϩ
(2)���д�ʩ�У������ڽ���������Ⱦ��Ũ�ȵ���__________��(����ĸ)
a���ڿ���ͨ�� b���ճ�������������Ȼ�����ú��ȼ��
c��ʹ�á���ɫ������װ�β��� d�����ڷ�һЩ����̼
(3) ������ЧӦ����ȫ���ע�Ļ�������֮һ��CO2��Ŀǰ�����к�����ߵ�һ���������塣��ˣ����ƺ�����CO2�ǽ������ЧӦ����Ч;������CO2ת�����л������Чʵ��̼ѭ����CO2ת�����л�������Ӻܶ࣬�磺
a��6CO2 + 6H2O
 C6H12O6+6O2 b��CO2 + 3H2
C6H12O6+6O2 b��CO2 + 3H2 CH3OH +H2O
CH3OH +H2Oc��CO2 + CH4
 CH3COOH d��2CO2 + 6H2
CH3COOH d��2CO2 + 6H2 CH2==CH2 + 4H2O
CH2==CH2 + 4H2O���Ϸ�Ӧ�У�����ܵ��� ��ԭ�������ʣ�ԭ��������=������������������������������֮�ȣ���ߵ��� ��
(4) ������β����Ⱦ���ѱ������˹�ע�������飨C8H8���������͵ijɷ֣�Ҫʹ����������ȫȼ�գ����������������������O2ռ1/5�����������ȣ���ͬ�����£�Ϊ ��������λС������
��

��ϰ��ϵ�д�
 �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д� �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�
�����Ŀ
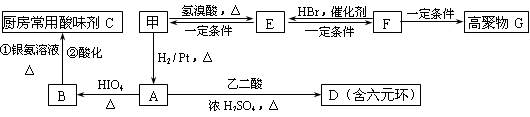
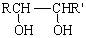
 RCHO + R��CHO
RCHO + R��CHO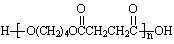 ������ƺ�����������Ϊԭ�ϣ����Լ���ѡ���ϳ�PBS���úϳ�·������ͼ��ʾ����ע����Ӧ��������
������ƺ�����������Ϊԭ�ϣ����Լ���ѡ���ϳ�PBS���úϳ�·������ͼ��ʾ����ע����Ӧ��������
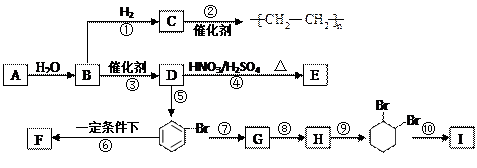
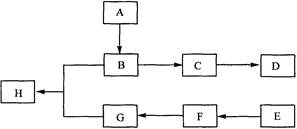
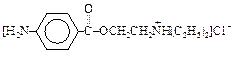
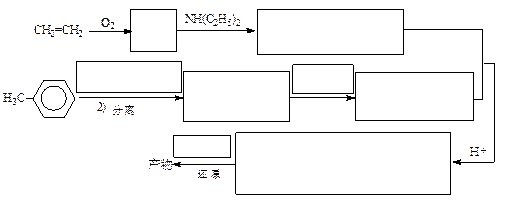
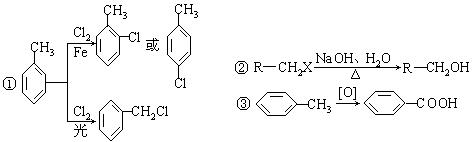
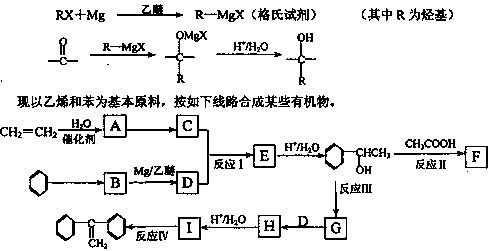
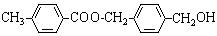 ����A�������ɷ���ͼʾ�е�һϵ�з�Ӧ������M���ڸ߷��ӻ����J��K��Ϊͬ���칹�壬N�IJ�������Ϊ����һ������ʯ�ͻ�����չˮƽ�ı�־��
����A�������ɷ���ͼʾ�е�һϵ�з�Ӧ������M���ڸ߷��ӻ����J��K��Ϊͬ���칹�壬N�IJ�������Ϊ����һ������ʯ�ͻ�����չˮƽ�ı�־��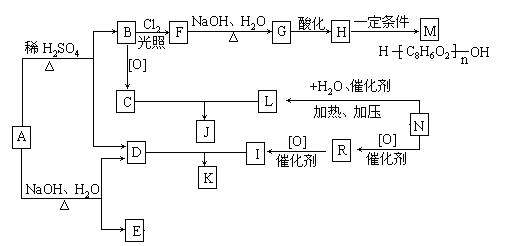
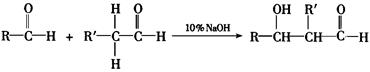
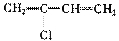 �ۺϵ�__________________________ _��
�ۺϵ�__________________________ _��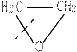 �����ߴ������ۺϵ�
�����ߴ������ۺϵ�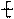 CH2CH2O
CH2CH2O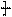 n����
n����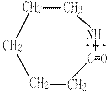 �����ۺϵ�_______________________________ ��
�����ۺϵ�_______________________________ ��