��Ŀ����
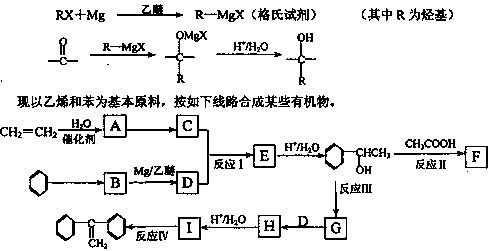
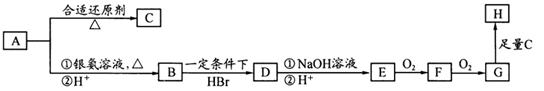
��14�֣���һ��ҩ��H�������õĿ��������ԣ���ϳ�·�����£�
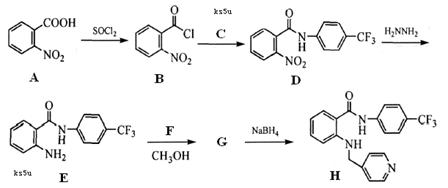
��֪��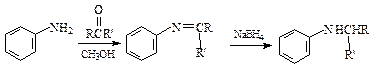
��Ӧ����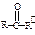 ��R��R����Ϊ��H��CH3��NH2�ȡ�
��R��R����Ϊ��H��CH3��NH2�ȡ�
��1��A��B�ķ�Ӧ������ �� ��
��2��д��C�Ľṹ��ʽ �� ��
��3������D����ˮ�ⷴӦ�ķ���ʽΪ �� ��
��4��д��E��G�ķ�Ӧ����ʽ �� ��
��5��д��������������������A��ͬ���칹��Ľṹ��ʽ �� ��
�������������ڱ����ϵ�һ�ȴ���������
��6��������������� ��һ�ֿ���������ҩ�������������Ϣ����
��һ�ֿ���������ҩ�������������Ϣ����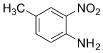 ��CH3COCl��CH3OHΪԭ�ϣ��ϳɹ��������Լ���ѡ���ϳ�·������ͼʾ�����£�
��CH3COCl��CH3OHΪԭ�ϣ��ϳɹ��������Լ���ѡ���ϳ�·������ͼʾ�����£�
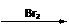 CH3CH2OH
CH3CH2OH  H2C��CH2 BrH2C��CH2Br
H2C��CH2 BrH2C��CH2Br
�� ��

��֪��
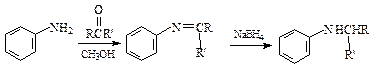
��Ӧ����
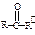 ��R��R����Ϊ��H��CH3��NH2�ȡ�
��R��R����Ϊ��H��CH3��NH2�ȡ���1��A��B�ķ�Ӧ������ �� ��
��2��д��C�Ľṹ��ʽ �� ��
��3������D����ˮ�ⷴӦ�ķ���ʽΪ �� ��
��4��д��E��G�ķ�Ӧ����ʽ �� ��
��5��д��������������������A��ͬ���칹��Ľṹ��ʽ �� ��
�������������ڱ����ϵ�һ�ȴ���������
��6���������������
 ��һ�ֿ���������ҩ�������������Ϣ����
��һ�ֿ���������ҩ�������������Ϣ���� ��CH3COCl��CH3OHΪԭ�ϣ��ϳɹ��������Լ���ѡ���ϳ�·������ͼʾ�����£�
��CH3COCl��CH3OHΪԭ�ϣ��ϳɹ��������Լ���ѡ���ϳ�·������ͼʾ�����£�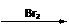 CH3CH2OH
CH3CH2OH 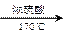 H2C��CH2 BrH2C��CH2Br
H2C��CH2 BrH2C��CH2Br�� ��
��1��ȡ����Ӧ��2�֣�
��2��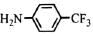 ��2�֣�
��2�֣�
��3��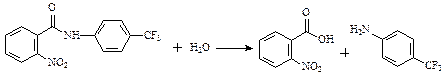 ��2�֣�
��2�֣�
��4��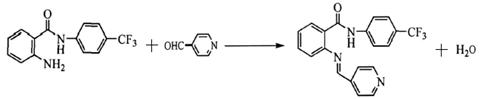 ��2�֣�
��2�֣�
��5��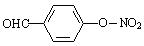 ��2�֣�
��2�֣�
��6��
��4�֣���������·��Ҳ�ɣ�
��2��
 ��2�֣�
��2�֣���3��
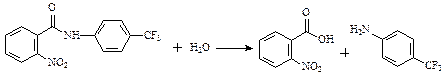 ��2�֣�
��2�֣���4��
 ��2�֣�
��2�֣���5��
 ��2�֣�
��2�֣���6��

��4�֣���������·��Ҳ�ɣ�
��

��ϰ��ϵ�д�
�����Ŀ
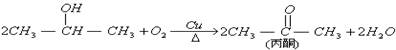
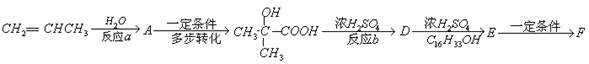 [FΪ�߾������ʽΪ
[FΪ�߾������ʽΪ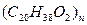 ]
]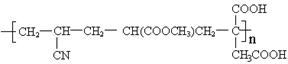

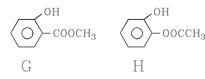 �� ��
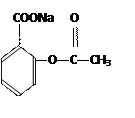
�� ��
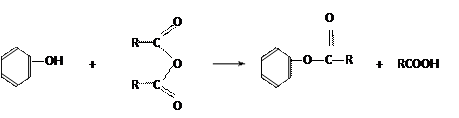
 ��
�� Һ�� ��
Һ�� ��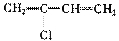 �ۺϵ�__________________________ _��
�ۺϵ�__________________________ _��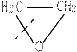 �����ߴ������ۺϵ�
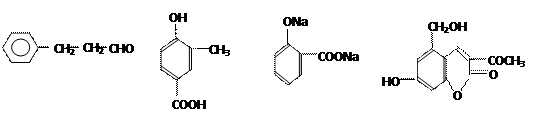
�����ߴ������ۺϵ�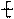 CH2CH2O
CH2CH2O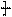 n����
n����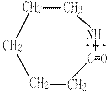 �����ۺϵ�_______________________________ ��
�����ۺϵ�_______________________________ ��