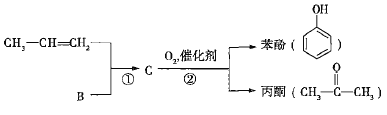
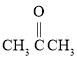��Ŀ����
����Ŀ��ʵ������Ҫ0.3 mol/L NaOH��Һ480 mL��1.0 mol/L������Һ250 mL��������������Һ����������ش��������⡣
��1����ͼ��ʾ��������������Һ�϶�����Ҫ����________(�����)������������Һ�����õ��IJ���������________(����������)��
��2�����ݼ�����������ƽ��ȡNaOH������Ϊ________g����ʵ����������������ȷ��������ʱ���ӿ̶��ߣ���������ҺŨ��________0.3 mol/L(����ڡ������ڡ���С�ڡ�����ͬ)����NaOH��Һ��ת��������ƿʱ����������������������ҺŨ��________0.3 mol/L��
��3�����ݼ����֪��������������Ϊ98%���ܶ�Ϊ1.84 g/cm3��Ũ��������Ϊ_______mL(����������һλС��)��
���𰸡�AC �ձ��������� 6.0 С�� С�� 13.6
��������
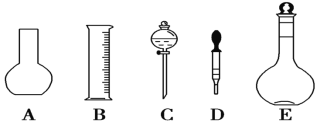
��1������һ�����ʵ���Ũ����Һ��һ�㲽��Ϊ�����㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��õ��������У�������ƽ��ҩ�ס��ձ���������������ƿ����ͷ�ιܣ������ò�����ͼʾ������ƽ����ƿ�ͷ�Һ©������AC������Ҫ�IJ�������Ϊ���ձ�����������
��2����Ҫ0.3mol/LNaOH��Һ480mL��Ӧѡ��500mL����ƿ����Ҫ���ʵ�������0.3mol/L��0.5L��40g/mol=6.0g������ʱ���ӿ̶��ߣ�������Һ���ƫ������![]() ��֪��ҺŨ��ƫ�ͣ���NaOH��Һ��ת��������ƿʱ���������������������ʵ����ʵ������٣���������ҺŨ��С��0.3 mol/L��
��֪��ҺŨ��ƫ�ͣ���NaOH��Һ��ת��������ƿʱ���������������������ʵ����ʵ������٣���������ҺŨ��С��0.3 mol/L��
��3����������Ϊ98%���ܶ�Ϊ1.84 g/cm3��Ũ��������ʵ���Ũ��Ϊc��![]() mol/L��18.4mol/L������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬������Ũ������������Ũ��������ΪxmL������xmL��18.4mol/L=250mL��1.0mol/L����ã�x��13.6��
mol/L��18.4mol/L������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬������Ũ������������Ũ��������ΪxmL������xmL��18.4mol/L=250mL��1.0mol/L����ã�x��13.6��

����Ŀ��![]() ʱ��������ĵ���ƽ�ⳣ�����£�
ʱ��������ĵ���ƽ�ⳣ�����£�
��ѧʽ |
|
| HClO |
����ƽ�ⳣ�� |
|
|
|
�ش��������⣺
��1��һ������£����¶�����ʱ��![]() ______����������������С��������������
______������������������������������
��2�������������ӽ�����������ɴ�С��˳����______�������
a��CO32- b��ClO- c��CH3COO- d��HCO3-
��3�����з�Ӧ���ܷ�������______�����
a. ![]()
b. ![]()
c. ![]()
d. ![]()
��4��������ˮϡ��![]() �Ĵ��ᣬ���и�ʽ��ʾ����ֵ��ˮ�������Ӷ��������______�������
�Ĵ��ᣬ���и�ʽ��ʾ����ֵ��ˮ�������Ӷ��������______�������
a. 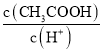 b.
b. ![]() c��
c��![]() d��
d��![]()
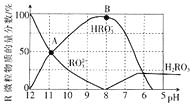
��5�������Ϊ10mLpH��Ϊ2�Ĵ�����Һ��HX��Һ�ֱ��ˮϡ����1000mL��ϡ������pH�仯��ͼ��ʾ��
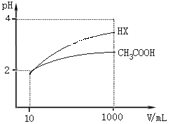
��HX�ĵ���ƽ�ⳣ��______������������������������С��������ͬ����ĵ���ƽ�ⳣ����ϡ�ͺ�HX��Һ��ˮ���������c��H+��______������Һ��ˮ���������c��H+����������___________��