��Ŀ����
��13�֣������������һ����Ҫ�Ļ�����Ʒ��ij��ȤС�����Ʊ���������ƾ��壨Na2S2O3��5H2O����
I��[��������]
��1��Na2S2O3��5H2O����ɫ�����壬������ˮ����ϡ��Һ��BaCl2��Һ����������ɡ�
��2����Na2CO3��Na2S���Һ��ͨ��SO2���Ƶ�Na2S2O3�����ò�Ʒ�г���������Na2SO3��Na2SO4��
��3��Na2SO3�ױ�������BaSO3������ˮ��������ϡHCl��
��.[�Ʊ���Ʒ]
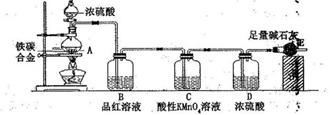
ʵ��װ����ͼ��ʾ��ʡ�Լг�װ�ã�
ʵ�鲽�裺
��1�����װ�������ԣ���ͼʾ�����Լ�������a��������____��E�е��Լ���___��ѡ��������ĸ��ţ���
A��ϡH2SO4 B��NaOH��Һ C������NaHSO3��Һ
��2������C����ƿ����Na2S��Na2CO3�����Һ������A����ƿ�μ�ŨH2SO4��
��3����Na2S��Na2CO3��ȫ���ĺ�����Ӧ������C�л��Һ����Һ��____����д�������ƣ����ᾧ�����ˡ�ϴ�ӡ�����õ���Ʒ��
��[̽���뷴˼]
��1��Ϊ��֤��Ʒ�к���Na2SO3��Na2SO4����С�����������ʵ�鷽�����뽫���������������������Լ���ϡHNO3��ϡH2SO4��ϡHCl������ˮ��ѡ��
ȡ������Ʒ���ϡ��Һ���μ�����BaCl2��Һ���а�ɫ�������ɣ�_____��������δ��ȫ�ܽ⣬���д̼�����ζ��������������ȷ����Ʒ�к���Na2SO3��Na2SO4��
��2��Ϊ����װ��C�����ɵ�Na2SO4�������ڲ��ı�ԭ��װ�õĻ����϶�ԭ��ʵ�鲽�裨2�������˸Ľ����Ľ���IJ�����_______��
��3��Na2S2O3��5H2O���ܽ�����¶����������������ò�Ʒͨ��_____�����ᴿ��
��1����Һ©����B ��3������
�� ��1�����ˣ�������ˮϴ�ӳ�����������м�������ϡHCl��
��2������A����ƿ�μ�Ũ���ᣬ���������彫װ���п����ž�������C����ƿ����Na2S��Na2CO3�����Һ����3���ؽᾧ
���������������.[�Ʊ���Ʒ]
��1�����������Ĺ����ص��֪������a�������Ƿ�Һ©��������װ�ÿ�֪��Aװ�����Ʊ�SO2�ģ�Cװ�����Ʊ���Na2S2O3��BDװ���Ƿ������ģ�����SO2�ж�����Ҫβ�����������Eװ��������SO2�ġ�����SO2���������������������������Һ���գ���E�е��Լ�������������Һ����ѡB��
��3������Na2S2O3��5H2O����ɫ�����壬������ˮ�����Ҫ����Һ�еõ���������ƾ��壬������Ҫ��������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����õ���Ʒ��
��[̽���뷴˼]
��1��Na2S2O3��5H2O��ϡ��Һ��BaCl2��Һ����������ɣ���ʵ��������а�ɫ�������ɣ����Ҫ��һ����֤����������ɫ�����еμ�ϡ���ᣬ������δ��ȫ�ܽ⣬���д̼�����ζ��������������ȷ����Ʒ�к���Na2SO3��Na2SO4��
��2���������������ױ��������������Σ���װ���к��п����������������������Σ�����Ϊ����װ��C�����ɵ�Na2SO4�������Ľ���Ĵ�ʩ������A����ƿ�μ�Ũ���ᣬ���������彫װ���п����ž�������C����ƿ����Na2S��Na2CO3�����Һ��
��3������Na2S2O3��5H2O���ܽ�����¶�������������������ò�Ʒͨ���ؽᾧ�����ᴿ��
���㣺���������Ʊ�ʵ�鷽�����������

����ʵ�鷽�����ܴﵽʵ��Ŀ�ĵ��ǣ�
| | ʵ��Ŀ�� | ʵ�鷽�� |
| A | ֤�������鷢����ȥ��Ӧ����ϩ���� | ���Թ��м����������������NaOH���Ҵ���Һ,����,����Ӧ����������ͨ��������Ȼ�̼��Һ |
| B | ֤��Mg(OH)2��������ת��ΪFe(OH)3���� | ��2 mL 1 mol/L NaOH��Һ���ȼ���3��1 mol/L MgCl2��Һ���ټ���3��1 mol/L FeCl3��Һ |
| C | ��������ˮ�������л�ԭ�� | ��������Һ�м��뼸��ϡ���ᣬˮԡ���ȼ����ӣ�Ȼ���������ϡNaOH��Һ���������м������Ƶ�������Һ����ˮԡ���� |
| D | ̽���¶ȶԻ�ѧƽ���Ӱ�� | ��NO2��������䡢��ˮ�У��۲���ɫ�ı仯 |
(12��)Ϊ���պ����÷Ͼ����ϼ��ᡰ��ɫ��Ⱦ����ij��ȤС���������̽����
| ʵ����� | ̽���Ͼ������ȷֽ����Ҫ����Ϊ�������Ļ���� |
| �������� | ��CuO�ܽ���������CO2��H2O�� �ڼױ��ɱ�����KMnO4��Һ����Ϊ�����ᣬ�����������ڱ��� |
| ʵ����� |   |
| ʵ���¼ | ����ͼ����װ�ã���������ǿ��װ��A�е��Թܣ�����װ���ڿ�����Ӱ�죩��һ��ʱ��ɹ۲쵽�������� ��Bװ���Թ�����Һ̬�������ɣ� ��C����ˮ����ɫ��dz�� ��E�к�ɫ����ͭ��죻 ��F����ˮ����ͭ������ |
��1��д��һ���������Ʊ��۱�ϩ��ѧ����ʽ ��
��2����������֪װ��B�Թ��е�Һ̬�����Ǽױ��ͱ��Ļ������ʵ���ȥ���к��еļױ� ��
��3��ʵ����װ��C�������� ��
��4����û��װ��D����ʵ����۲�����Ӱ���� ��
��5����ͬѧ��Ϊ��E�к�ɫ����ͭ��죬F����ˮ����ͭ�������ǷϾɾ۱�ϩ�����ȷֽ�����к��������µġ�������Fװ�ú�����һ��ʵ��װ�ã���ȷ��ʹCuO�������̬���������������������ӵ�װ��ͼ��Ҫע������ʢ�ŵ��Լ����ƣ��������߿��ڡ�


 H2O + CH3CH2��O��CH2CH3 (����)
H2O + CH3CH2��O��CH2CH3 (����)
 NH2COONH4(s) ��H��0
NH2COONH4(s) ��H��0