��Ŀ����
����Ŀ������ʵ������Ҫ0.1 mol��L1NaOH��Һ500 mL��������Һ����������ش��������⡣
��1������ͼ��ʾ�����У�����������Һ�϶�����Ҫ����_________�������������ͼ�����������⣬����������Һ����Ҫ�IJ���������__________________��

��2������ʱ������ȷ�IJ���˳����__________������ĸ��ʾ��ÿ������ֻ��һ������
A��������ˮϴ���ձ�2�Ρ�3�Σ�ϴ��Һ��ע������ƿ����
B����ʢ��NaOH������ձ��м�������ˮ�ܽ�
C�����ձ�������ȴ����Һ�ز�����ע������ƿ��
D��������ƿ�ǽ����������µߵ���ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ��Һ��ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1 cm��2 cm��
��3����������ƿ��������������������ȷŨ����Һ��������������������Һ���������������ȣ�
��ʹ��֮ǰҪ����Ƿ�©ˮ����Щ��������ȷ����__________������ĸ����
A���٢ڢۢ� B���ڢ� C���٢ڢ� D���ڢۢ�
��4�����ݼ�����������ƽ��ȡ��NaOH��������Ϊ______g��
��������1 mol/L��ϡ������Һ500 mL���ش��������⡣
��5����Ҫ��������Ϊ98%���ܶ�Ϊ1.84 g/cm3��Ũ��������Ϊ_________mL������������һλС���������ʵ������25 mL��50 mL��10 0mL��Ͳ��Ӧѡ��________mL������Ͳ��á�
��6����������������ϡ������ҺŨ��ƫ�ߵ���______������ĸ����
A���ܽ����Һû����ȴ�����¾�ת��
B��ת��ʱû��ϴ���ձ���������
C��������ƿ��ˮ����ʱ�۾�����Һ��
D������Ͳ��ȡŨ�����ϴ����Ͳ����ϴ��Һת�Ƶ�����ƿ
E��ҡ�Ⱥ���Һ����ڿ̶��ߣ��ּ�����ˮ���̶���
���𰸡�������1��bd��1�֣� 500 mL����ƿ����ͷ�ιܣ�1�֣�
��2��BCAFED��2�֣�
��3��A��2�֣�
��4��2.0��2�֣�
������5��27.2��2�֣� 50��1�֣�
��6��ACD��3�֣�
����������.��1��ʵ������Ҫ0.1 mol��L1NaOH��Һ500 mL���������ƹ��̿�����Ҫ��������������ƽ���ձ�����������500 mL����ƿ�ͽ�ͷ�ιܣ�����Ҫ���Ƿ�Һ©����©����
��2�����ƹ���һ���Ǽ��㡢�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ������ݺ�ҡ�ȵȡ���������ʱ������ȷ�IJ���˳����BCAFED��
��3��������ƿ������ȷŨ����Һ����������ȷ��������ƿ����������Һ����ȷ��������ƿ�����������ȣ���ȷ��������ƿʹ��֮ǰҪ����Ƿ�©ˮ����ȷ����ѡA��
��4�����ݼ�����������ƽ��ȡ��NaOH��������Ϊ0.5 L��0.1 mol/L��40 g/mol=2.0 g��
��.��5��������1 mol/L��ϡ������Һ500 mL������Ҫ������������ʵ���Ϊ0.5 mol��������49 g�������Ҫ��������Ϊ98%���ܶ�Ϊ1.84 g/cm3��Ũ��������Ϊ![]() ��27.2 mL�����ѡ��50 mL��Ͳ��á�
��27.2 mL�����ѡ��50 mL��Ͳ��á�
��6��A���ܽ����Һû����ȴ�����¾�ת�ƣ�����ȴ����Һ������٣�Ũ��ƫ�ߣ�B��ת��ʱû��ϴ���ձ��������������ʼ��٣�Ũ��ƫ�ͣ�C��������ƿ��ˮ����ʱ�۾�����Һ�棬����Һ������٣�Ũ��ƫ�ߣ�D������Ͳ��ȡŨ�����ϴ����Ͳ����ϴ��Һת�Ƶ�����ƿ�����������ӣ�Ũ��ƫ�ߣ�E��ҡ�Ⱥ���Һ����ڿ̶��ߣ��ּ�����ˮ���̶��ߣ���Һ������ӣ�Ũ��ƫ�ͣ���ѡACD��

 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
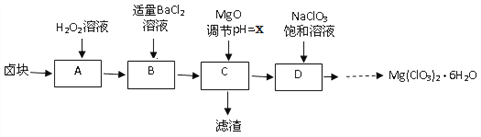
�����߿����ϵ�д�����Ŀ��ʵ������±�飨��Ҫ�ɷ�ΪMgCl2��6H2O������MgSO4.FeCl2�����ʣ��Ʊ�����Mg(ClO3)2��6H2O���������£�

��֪�������ֻ�������ܽ��(S)���¶�(T)�仯������ͼ��ʾ��
������ʱһЩ���ʵ�Ksp���±���
��ѧʽ | Fe(OH)2 | Fe(OH)3 | Mg(OH)2 |
Ksp | 8.0��10-16 | 8.0��10-38 | 1.8x10-11 |
��Mg(ClO3)2�н�ǿ�������ԣ��仹ԭ������Cl-.
��1��H2O2�ĵ���ʽΪ_________
��2�������ijɷ���____________���ѧʽ����
��3�����ⶨ��D�������ӵ�Ũ��Ϊ1��10-5 mol/L,��xΪ______
��4��D���������Ļ�ѧ��Ӧ����ʽΪ_____����ͼ����D��......����Mg(ClO3)2��6H2O�����ʵ�鲽������Ϊ���ټ�����������_______���벹�䣩������ȴ�ᾧ���ܹ���ϴ�ӡ�
��5����Ʒ��Mg(ClO3)2��6H2O�����IJⶨ��
����1��ȷ����3.50 g��Ʒ���100 mL��Һ��
����2��ȡ10.00 mL��Һ����ƿ�У�����10.00 mLϡ�����20 .00mL 1.000 mol/L��FeSO4��Һ���ȡ�
����3����ȴ�����£���0.100 mol/L K2Cr2O7��Һ�ζ�ʣ���Fe2�����յ㡣
����4��������2��3�ظ�����
�ٲ���3�з�����Ӧ�����ӷ���ʽ____________
�ڲ���3�����ζ�ǰ���ñ�Һ��ϴ�ζ��ܣ����ᵼ�����ս��_____������ƫ����. ��ƫС����������������
����ƽ������K2Cr2O7��Һ15.00 mL�����Ʒ��Mg(ClO3)2��6H2O����M=299g/mol������������Ϊ___________
����Ŀ��������Ļ������ڿ��С������ѧ��ҵ�о�����Ҫ��Ӧ�á�
��1����֪��SO2(g)+1/2O2(g)=SO3(g) H=��99kJ��mol-1
SO2(g)+NO2(g)=SO3(g)+ NO(g) H=��41.8kJ��mol-1
CO(g)+1/2O2(g)=CO2(g) H=��283kJ��mol-1
��д��CO��NO2��Ӧ����CO2��NO���Ȼ�ѧ��Ӧ����ʽ_________________,1molCO��1molNO2��Ӧ����CO2��NO��������Ӧʾ��ͼ�е�E2=_______kJ��mol-1

��2����ͨ����Ӧ2CO(g)+SO2(g)![]() 2CO2(g)+S(l)��������¯�̵����е��ж�����,ij�¶������ܱ�������ͨ��һ������SO2��CO���巢����Ӧ��5���Ӻ��ƽ�⣬����0.5 mol/L��CO2��
2CO2(g)+S(l)��������¯�̵����е��ж�����,ij�¶������ܱ�������ͨ��һ������SO2��CO���巢����Ӧ��5���Ӻ��ƽ�⣬����0.5 mol/L��CO2��
�ٷ�Ӧ��ʼ��ƽ��ʱ,SO2��ƽ����Ӧ����v(SO2)=________��
��������������ʱ��SO2��ƽ��ת�����淴Ӧ�¶ȱ仯��ͼ�����ü�����ֽ���ԭ��_________________________________________;

��3��25�棬��0.10 molL1H2S��Һ�У���ҺpH��c(S2)��ϵ���±���������Һ����ı仯��H2S�Ļӷ���,ij��Һ��0.020 molL1Mn2+��0.1 molL1H2S������Һ��pH=5ʱ��
Mn2+��ʼ��������MnS���ܶȻ�Ksp =________________;
pH | 3 | 5 | 7 | 9 | 11 |
c(S2-)/ molL1 | 1.4��1015 | 1.4��1011 | 6.8��108 | 1.3��105 | 1.3��103 |
��4��һ���ѳ���ˮ��NH4�� �ĵ绯ѧװ����ͼ��ʾ����д�������ĵ缫��Ӧ����ʽ��____________, ���һ��ʱ���������Χ��Һ��pH_____(�����, ����С�����䡱)��
