��Ŀ����
��8�֣�ij��ѧ��ȤС��Ϊ��̽��п��Ũ���ᷴӦ��������ijɷ���������ʵ�飺
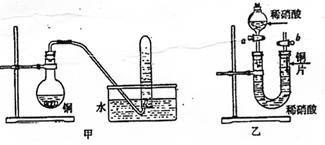
��50gп����50mLŨH2SO4�ڼ��������³�ַ�Ӧ��п����ʣ�࣬�ռ���һ���������
�壬���������������ɱ�״��Ϊ11.2L��
��1����ѧ��ȤС�����Ƶõ�����X�л��е���Ҫ�������������__________���������ֽ������Ҫԭ���ǣ������ӷ���ʽ��ʾ��_________________ ��
��2��ʵ����֤��Ϊ�˼�����Ҫ��������ijɷ֣���ѧ��ȤС���ͬѧ���������ʵ�飬������Xȡ������̽����
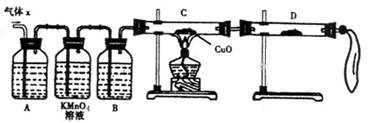
��A�м�����Լ�������__________��B�м�����Լ�������_________��
�ڹ۲쵽C�е�ʵ��������____________________��D�����ѡ����Լ���________��
��3�����۷���������С��ͬѧ�ռ������������Ϊ25.8g������Ũ��������ʵ���Ũ��Ϊ18.0mol/L����ͨ������ȷ������X�и��ɷ����ʵ����ֱ�Ϊ_____________��
��50gп����50mLŨH2SO4�ڼ��������³�ַ�Ӧ��п����ʣ�࣬�ռ���һ���������
�壬���������������ɱ�״��Ϊ11.2L��
��1����ѧ��ȤС�����Ƶõ�����X�л��е���Ҫ�������������__________���������ֽ������Ҫԭ���ǣ������ӷ���ʽ��ʾ��_________________ ��
��2��ʵ����֤��Ϊ�˼�����Ҫ��������ijɷ֣���ѧ��ȤС���ͬѧ���������ʵ�飬������Xȡ������̽����

��A�м�����Լ�������__________��B�м�����Լ�������_________��
�ڹ۲쵽C�е�ʵ��������____________________��D�����ѡ����Լ���________��
��3�����۷���������С��ͬѧ�ռ������������Ϊ25.8g������Ũ��������ʵ���Ũ��Ϊ18.0mol/L����ͨ������ȷ������X�и��ɷ����ʵ����ֱ�Ϊ_____________��
(8��)��1��H2��1�֣���Zn+2H+��Zn2++H2����1�֣�
��2����NaOH��Һ��1�֣� ŨH2SO4��1�֣��ں�ɫ�����죨1�֣�����ˮCuSO4��1�֣�
��3��SO2Ϊ0.4mol��H2Ϊ0.1mol��2�֣�
��2����NaOH��Һ��1�֣� ŨH2SO4��1�֣��ں�ɫ�����죨1�֣�����ˮCuSO4��1�֣�
��3��SO2Ϊ0.4mol��H2Ϊ0.1mol��2�֣�
��

��ϰ��ϵ�д�
 �Ͻ�ƽ��У����ϵ�д�
�Ͻ�ƽ��У����ϵ�д�
�����Ŀ








 ��IBr ��
��IBr �� ��IBr��KI��I2��KBr ��I2��2S2O32����2I����S4O62��
��IBr��KI��I2��KBr ��I2��2S2O32����2I����S4O62��