��Ŀ����
��1����������(N2H4)Ϊȼ�ϣ������������������������߷�Ӧ���ɵ�������̬ˮ��
��֪��N2(g)��2O2(g)��N2O4(g) ��H��+10.7kJ��mol-1
N2H4(g)��O2(g)��N2(g)��2H2O(g) ��H��-543kJ��mol-1
д����̬�º�N2O4��Ӧ���Ȼ�ѧ����ʽ�� ��
����֪�����������ڴ����л��ڽϸ��¶��º����ȶ����ڣ��������ת��Ϊ�������������ƶ��ɶ���������ȡ�����������ķ�Ӧ����(���ʩ)�� ��
��2����ѧ�������һ��ʹ�ù������ʵ�ȼ�ϵ�أ���Ч�ʸ��ߣ������ں��캽�ա�
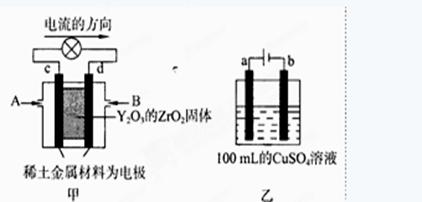
ͼ����ʾװ���У���ϡ����������Ϊ���Ե缫���������Ϸֱ�ͨ��CH4�Ϳ��������й��������Dz�����Y2O3��ZrO2���壬���ڸ������ܴ����������ɵ�O2-(O2+4e ��2O2-)
��c�缫Ϊ ��d�缫�ϵĵ缫��ӦʽΪ ��
��ͼ���ǵ��100mL 0.5mol��L-1 CuSO4��Һ��a�缫�ϵĵ缫��ӦʽΪ ����a�缫����56mL(��״��)���壬��������Һ��pH= (��������Һ����仯)����Ҫʹ�������Һ�ָ������ǰ��״̬���ɼ��� (ѡ����ĸ���)
a.CuO b.Cu(OH)2 c.CuCO3 d.Cu2(OH)2CO3
��֪��N2(g)��2O2(g)��N2O4(g) ��H��+10.7kJ��mol-1
N2H4(g)��O2(g)��N2(g)��2H2O(g) ��H��-543kJ��mol-1
д����̬�º�N2O4��Ӧ���Ȼ�ѧ����ʽ�� ��
����֪�����������ڴ����л��ڽϸ��¶��º����ȶ����ڣ��������ת��Ϊ�������������ƶ��ɶ���������ȡ�����������ķ�Ӧ����(���ʩ)�� ��
��2����ѧ�������һ��ʹ�ù������ʵ�ȼ�ϵ�أ���Ч�ʸ��ߣ������ں��캽�ա�

ͼ����ʾװ���У���ϡ����������Ϊ���Ե缫���������Ϸֱ�ͨ��CH4�Ϳ��������й��������Dz�����Y2O3��ZrO2���壬���ڸ������ܴ����������ɵ�O2-(O2+4e ��2O2-)
��c�缫Ϊ ��d�缫�ϵĵ缫��ӦʽΪ ��
��ͼ���ǵ��100mL 0.5mol��L-1 CuSO4��Һ��a�缫�ϵĵ缫��ӦʽΪ ����a�缫����56mL(��״��)���壬��������Һ��pH= (��������Һ����仯)����Ҫʹ�������Һ�ָ������ǰ��״̬���ɼ��� (ѡ����ĸ���)
a.CuO b.Cu(OH)2 c.CuCO3 d.Cu2(OH)2CO3
��14�֣�
��1����2 N2H4(g) + N2O4(g)= 3N��(g)��4H20(g) ��H����1096.7KJ��mol-1��2�֣�
�ڼ�ѹ�����£���1�֣�
��2����������2�֣� CH4 - 8e- + 402-=CO2+2H2O ��2�֣�
��4OH��- 4e-=2H2O+O2 ��2�֣� 1 ��2�֣� a��c ��2�֣�
��1����2 N2H4(g) + N2O4(g)= 3N��(g)��4H20(g) ��H����1096.7KJ��mol-1��2�֣�
�ڼ�ѹ�����£���1�֣�
��2����������2�֣� CH4 - 8e- + 402-=CO2+2H2O ��2�֣�
��4OH��- 4e-=2H2O+O2 ��2�֣� 1 ��2�֣� a��c ��2�֣�
�����������1����a��N2��g��+2O2��g��=N2O4��g����H=10.7kJ��mol��1��b��N2H4��g��+O2��g��=N2��g��+2H2O��g����H=-543kJ��mol��1
���ݸ�˹����b��2-a�õ� 2N2H4��g��+N2O4��g��=3N2��g��+4H2O��g����H=-1096.7KJ��mol��1��
����2N2H4��g��+N2O4��g��=3N2��g��+4H2O��g����H=-1096.7KJ��mol��1��
�������������ڴ����л��ڽϸ��¶��º����ȶ����ڣ���������ת��Ϊ�����������ɶ���������ȡ������������2NO2
 N2O4����Ӧ�Ƿ��ȷ�Ӧ����Ӧǰ�����������С���Է�Ӧ����Ϊ������ѹǿ�����¶������ڷ�Ӧ������У�
N2O4����Ӧ�Ƿ��ȷ�Ӧ����Ӧǰ�����������С���Է�Ӧ����Ϊ������ѹǿ�����¶������ڷ�Ӧ������У��ʴ�Ϊ������ѹǿ�����£�
��2����ͼ1��ԭ��أ����ݵ��������Ǵ�����������c�缫Ϊ�����������õ����ӷ�����ԭ��Ӧ��d�缫Ϊ��ظ��������ǵ��ӷ�����ԭ��Ӧ���������Ϸֱ�ͨ��CH4�Ϳ��������й��������Dz�����Y2O3��ZrO2���壬���ڸ������ܴ����������ɵ�O2�����ӣ���ϵ����غ�д���缫��ӦΪ��CH4- 8e-+4O2��=CO2+2H2O��
���������� CH4- 8e-+4O2��=CO2+2H2O��
����ͼ2��ʾ���100mL0.5mol?L-1CuSO4��Һ�������ĵ��ط�ӦΪ��2CuSO4+2H2O
 2Cu+O2��+2H2SO4�����Դ����������Ϊ��������Һ�� �����������ǵ��ӷ���������Ӧ���缫��ӦΪ��4OH��-4e��=2H2O+O2������a�缫����56mL����״��������Ϊ���������ʵ���Ϊ0.0025mol�������������������ʵ���Ϊ0.01mol����Һ���������������ʵ���Ϊ0.01mol��c��H����=
2Cu+O2��+2H2SO4�����Դ����������Ϊ��������Һ�� �����������ǵ��ӷ���������Ӧ���缫��ӦΪ��4OH��-4e��=2H2O+O2������a�缫����56mL����״��������Ϊ���������ʵ���Ϊ0.0025mol�������������������ʵ���Ϊ0.01mol����Һ���������������ʵ���Ϊ0.01mol��c��H����=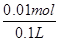 =0.1mol��L��1��PH=-lg0.1=1����������Һ��������CuSO4��Һÿ��ʧ2��Cuԭ�ӣ�����ʧ2�� Oԭ�ӣ��൱����ʧһ��CuO��Ϊ��ʹCuSO4��Һ���ָ�ԭŨ�ȣ�Ӧ����CuO��Ҳ���Լ���CuCO3�����ϻָ���ҺŨ�ȵĶ�����ϵ�������ܼ���Cu��OH��2��Cu2��OH��2CO3����ΪCuCO3+H2SO4
=0.1mol��L��1��PH=-lg0.1=1����������Һ��������CuSO4��Һÿ��ʧ2��Cuԭ�ӣ�����ʧ2�� Oԭ�ӣ��൱����ʧһ��CuO��Ϊ��ʹCuSO4��Һ���ָ�ԭŨ�ȣ�Ӧ����CuO��Ҳ���Լ���CuCO3�����ϻָ���ҺŨ�ȵĶ�����ϵ�������ܼ���Cu��OH��2��Cu2��OH��2CO3����ΪCuCO3+H2SO4 CuSO4+CO2��+H2O���൱�ڼ�CuO����Cu��OH��2+H2SO4
CuSO4+CO2��+H2O���൱�ڼ�CuO����Cu��OH��2+H2SO4 CuSO4+2H2O��Cu2��OH��2CO3+2H2SO4=2CuSO4 +CO2��+3H2O�������������������ˮ��ѡac��
CuSO4+2H2O��Cu2��OH��2CO3+2H2SO4=2CuSO4 +CO2��+3H2O�������������������ˮ��ѡac����Ϊ��4OH��- 4e-=2H2O+O2 ��1��ac��

��ϰ��ϵ�д�
�����Ŀ
 cC(��)+dD(��)����H��Q������ͼ�ش�
cC(��)+dD(��)����H��Q������ͼ�ش�


 4CO(g)+BaS(s) ��H1 = +571.2kJ/mol ��
4CO(g)+BaS(s) ��H1 = +571.2kJ/mol ��

 CO(NH2)2(l)+H2O(g)��
CO(NH2)2(l)+H2O(g)��