��Ŀ����
�����£���16 mol N2��24 mol H2�Ļ������ͨ��һ���̶��ݻ�Ϊ4L���ܱ������У��������·�Ӧ��N2��g��+3H2��g��![]() 2NH3��g����10���Ӻ�Ӧ��ƽ��ʱ��NH3�ĺ��������������Ϊ25%�����еĹ�˵����ȷ���� �� ��
2NH3��g����10���Ӻ�Ӧ��ƽ��ʱ��NH3�ĺ��������������Ϊ25%�����еĹ�˵����ȷ���� �� ��
A���ﵽƽ��ʱ��N2��H2��ת����֮��Ϊ1��1
B��10������v(H2)=0.35mol/(L��min)
C��ƽ����������ܶ�Ϊ128g/L
D��ƽ���������У�n��N2����n��H2����n��NH3��=1��3��2
C
����:
N2��g��+3H2��g��![]() 2NH3��g��
2NH3��g��
��㣺 16 24 0
�仯����x 3x 2x
ƽ��ʱ��16-x 24-3x 2x
��ƽ��ʱ��NH3�ĺ���Ϊ25%��֪x=4mol.����һ������֪��ֻ��C��ȷ

��ϰ��ϵ�д�
�����Ŀ
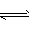 2NH3��g����10���Ӻ�Ӧ��ƽ��ʱ��NH3�ĺ��������������Ϊ25%�����еĹ�˵����ȷ����
2NH3��g����10���Ӻ�Ӧ��ƽ��ʱ��NH3�ĺ��������������Ϊ25%�����еĹ�˵����ȷ����