��Ŀ����
�����£���16 mol N2��24 mol H2�Ļ������ͨ��һ���̶��ݻ�Ϊ4L���ܱ������У��������·�Ӧ��N2��g��+3H2��g��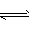 2NH3��g����10���Ӻ�Ӧ��ƽ��ʱ��NH3�ĺ��������������Ϊ25%�����еĹ�˵����ȷ����
2NH3��g����10���Ӻ�Ӧ��ƽ��ʱ��NH3�ĺ��������������Ϊ25%�����еĹ�˵����ȷ����
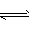 2NH3��g����10���Ӻ�Ӧ��ƽ��ʱ��NH3�ĺ��������������Ϊ25%�����еĹ�˵����ȷ����
2NH3��g����10���Ӻ�Ӧ��ƽ��ʱ��NH3�ĺ��������������Ϊ25%�����еĹ�˵����ȷ���� [ ]
A���ﵽƽ��ʱ��N2��H2��ת����֮��Ϊ1��1
B��10������v(H2)=0.35mol/(L��min)
C��ƽ����������ܶ�Ϊ128g/L
D��ƽ���������У�n��N2����n��H2����n��NH3��=1��3��2
B��10������v(H2)=0.35mol/(L��min)
C��ƽ����������ܶ�Ϊ128g/L
D��ƽ���������У�n��N2����n��H2����n��NH3��=1��3��2
C

��ϰ��ϵ�д�
�����Ŀ