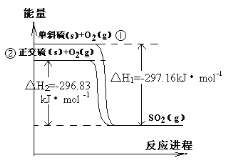
��Ŀ����
����Ŀ����ԭ�������ĵ��������ֶ�����Ԫ�أ�����ĸ��ʾ��ԭ�Ӱ뾶����Դ�С��������ۻ�����۵ı仯��ͼ��ʾ�������жϳ���Ԫ�ػش����⣺
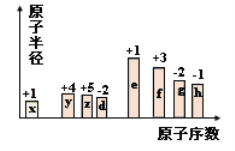
��1��f��Ԫ�����ڱ���λ����_______��
��2������Ԫ���γɵļ������������У����Ӱ뾶�����ǣ��û�ѧʽ��ʾ����ͬ��_______________����e��f��g��h����Ԫ�ص�����������Ӧ��ˮ�����е�������ǿ����___________________��
��3��d��e���γ�ԭ�Ӹ�����Ϊ1��1�Ļ�����û�����ĵ���ʽΪ________________��0.1 mol�û�����������ˮ��Ӧʱת�Ƶĵ�����Ϊ____________��
���𰸡���3���ڣ���A�� S2�� HClO4 ![]() 6.02��1022��0.1NA
6.02��1022��0.1NA
��������
����Ԫ�ض��Ƕ�����Ԫ�أ�����Ԫ�������ɣ�����������Ԫ�طֱ�ΪH��C��N��O��Na��Al��S��Cl���ݴ˷�����
ͬ���ڴ�������ԭ�Ӱ뾶���μ�С������Ԫ����������ϼ�һ�����������������۵���8��������������ͼ�еĻ��ϼۡ�ԭ�Ӱ뾶�Ĵ�С��ԭ���������Ƴ����ֶ�����Ԫ�طֱ�ΪH��C��N��O��Na��Al��S��Cl��
��1��fΪAl��λ�ڵ������ڢ�A�壻
��Ϊ�������ڢ�A�壻
��2�����Ӳ�ṹ��ͬ�����ӣ��˵����Խ�����Ӱ뾶ԽС�����Ӳ���һ��Խ�࣬�뾶Խ�����Ӱ뾶������S2�����ǽ�����Խǿ��������������Ӧˮ���������Խǿ��ͬ���ڴ������ҷǽ�������ǿ��������������Ӧ��ˮ�����������ǿ����HClO4��
��ΪS2����HClO4��
��3��d��e�γɵĻ�������ԭ�Ӹ�����Ϊ1��1������������Na2O2�������ʽΪ![]() ������������ˮ��Ӧ�ķ���ʽΪ2Na2O2��2H2O=4NaOH��O2����2molNa2O2��ˮ��Ӧ��ת�Ƶ������ʵ���Ϊ2mol����0.1mol����������H2O��Ӧת�Ƶ������ʵ���Ϊ0.1mol��������Ϊ6.02��1022��0.1NA��
������������ˮ��Ӧ�ķ���ʽΪ2Na2O2��2H2O=4NaOH��O2����2molNa2O2��ˮ��Ӧ��ת�Ƶ������ʵ���Ϊ2mol����0.1mol����������H2O��Ӧת�Ƶ������ʵ���Ϊ0.1mol��������Ϊ6.02��1022��0.1NA��
����![]() ��6.02��1022��0.1NA��
��6.02��1022��0.1NA��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ij������Һ�к����±������е�һ�ֻ���֡��ֱ�ȡ����ˮ��Һ���ݽ���ʵ�飬������£�
������ | Fe2+��Fe3+��Al3+ |
������ | SO32-��CO32-��SiO32-��I����NO3- |
����һ����Һ�м���������ᣬ�������ݣ���Һ��ɫ������Գ��壻
�ڼ��������Һ�м����������Ȼ�̼�������ã��²�����Ϻ�ɫ���ϲ���Һ���ֻ�ɫ��
������һ����Һ�м����������������Һ����������������������Һ�������ϵ��ͼ��ʾ��
����������Ϣ���ش��������⣺
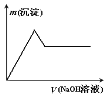
��1��ԭ��Һ�п϶����е�������______________________________________________��
��2���������ɵ�������______________(�ѧ����)�������е�������_____________������ĸ��ţ���
A����ɫ��ζ B���ܱ�NaOH��Һ���� C�����ڴ�����Ⱦ�� D��������ˮ
��3�����з�����Ӧ�����ӷ���ʽ��_______________________________________________��
��4�����г����ܽ�Ļ�ѧ��Ӧ����ʽ��________________________________________��
����Ŀ��A��B�����л�����Ի��ܣ��й��������£�
���� | �ܶ�(g��cm-3) | �۵�/�� | �е�/�� | �ܽ��� |
A | 0.7893 | -117.3 | 78.5 | ��ˮ������Ȼ��� |
B | 0.7137 | -116.6 | 34.5 | ������ˮ |
(1)Ҫ��ȥA��B�Ļ�����е�����B���ɲ��õ�_______________�����ɵõ�A��
A.���� B.�ؽᾧ C.��ȡ D.��ˮ�������Һ
(2)���л���A�����������г��ȼ�գ�A������ǡ����ȫ��Ӧ������6.72L(��״��)����������5.4gH2O��8.8gCO2��������ʵ�ʵ��ʽ��__________������ͼ��ʾ��A����Է�������Ϊ46������֪�л���A�ĺ˴Ź���������ͼ��ʾ����A�Ľṹ��ʽΪ________________��
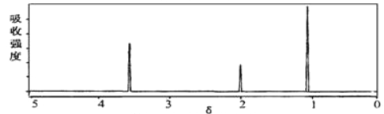
(3)��ͼ��B������ͼ��������Է�������Ϊ ________ ��
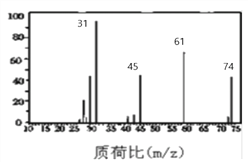
(4)B�ĺ��������ͼ��ʾ����B�Ľṹ��ʽΪ__________________________��
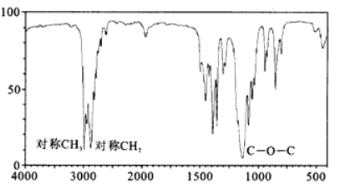
(5)ȷ��ȡһ��������A��B�Ļ����������������ȼ�գ�����������ͨ����������ˮ�Ȼ��ƺͼ�ʯ�ң����������ֱ�����14.4g��26.4g������������A��B�����ʵ���֮��_____________________��