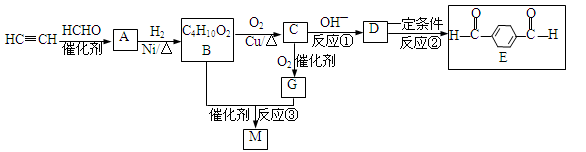
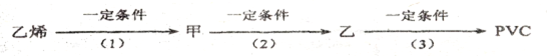��Ŀ����
ij�������Ա�Ϊ��Ҫԭ�ϣ���������·�ߺϳ�����ҩ�������ӡ�
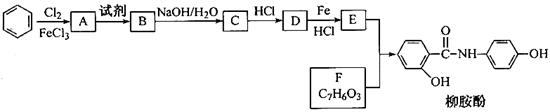
��֪�� ����ش��������⣺
����ش��������⣺
��1�����������ӣ�����˵����ȷ���� ��
��2��д��A��B��Ӧ������Լ� ��
��3��д��B��C�Ļ�ѧ����ʽ ��
��4��д��������F�Ľṹ��ʽ ��
��5��д��ͬʱ��������������F��ͬ���칹��Ľṹ��ʽ ��д��3�֣���
�����������ұ����������ֲ�ͬ��ѧ��������ԭ�ӣ����ܷ���������Ӧ��
��6���Ա�����ϩΪԭ�Ͽɺϳɾ۱���ϩ������ƺϳ�·�ߣ����Լ����ܼ���ѡ����
ע���ϳ�·�ߵ���д��ʽ��������ʵ������ͼ��
CH3CHO CH3COOH
CH3COOH CH3COOCH2CH3
CH3COOCH2CH3

��֪��
 ����ش��������⣺
����ش��������⣺��1�����������ӣ�����˵����ȷ���� ��
| A��1 mol�����������Ժ�2 molNaOH��Ӧ | B��������������Ӧ |
| C���ɷ���ˮ�ⷴӦ | D�������巢��ȡ����Ӧ |
��3��д��B��C�Ļ�ѧ����ʽ ��
��4��д��������F�Ľṹ��ʽ ��
��5��д��ͬʱ��������������F��ͬ���칹��Ľṹ��ʽ ��д��3�֣���
�����������ұ����������ֲ�ͬ��ѧ��������ԭ�ӣ����ܷ���������Ӧ��
��6���Ա�����ϩΪԭ�Ͽɺϳɾ۱���ϩ������ƺϳ�·�ߣ����Լ����ܼ���ѡ����
ע���ϳ�·�ߵ���д��ʽ��������ʵ������ͼ��
CH3CHO
 CH3COOH
CH3COOH CH3COOCH2CH3
CH3COOCH2CH3��1��CD
Ũ�����Ũ����
��3��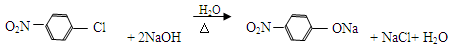
��4��������
��5��
��6��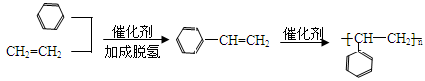
Ũ�����Ũ����
��3��
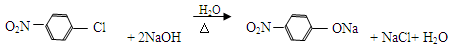
��4��������
��5��

��6��

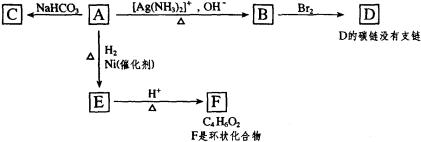
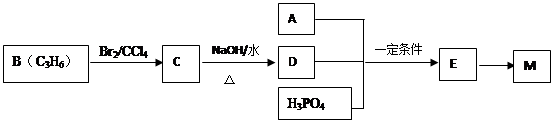
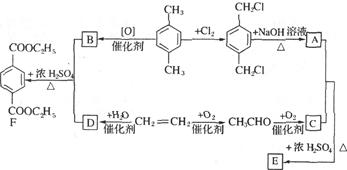
���ݺϳ�·�ߣ��������ӵķ��ӽṹ�����Ƴ�FΪ���ǻ�������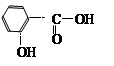
EΪ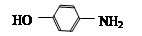 ��DΪ
��DΪ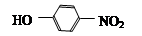 ��CΪ
��CΪ ��BΪ
��BΪ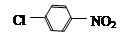 ��AΪ
��AΪ ��
��
��1�������ӷ����к����ǻ������������ԡ�������(�������ļ�)��������ˮ�ⷴӦ��1 mol������������ ��3 mol NaOH��Ӧ�������Ͽ��Է���������Ӧ�������Ϸ��ǻ��ڶ�λ������Է��������Ӧ��1mol��������Br2��Ӧ����������4 molBr2��
��2����������������������뷴Ӧ������Ũ������������ ��ԭ��ˮ������1molNaOH�������зӻ�����������1molNaOH������ 2molNaOH������д����ػ�ѧ����ʽ��
��ԭ��ˮ������1molNaOH�������зӻ�����������1molNaOH������ 2molNaOH������д����ػ�ѧ����ʽ��
���ǻ������ᣬ�������⣬����һ���Ȼ���һ���ǻ�������ĿҪ��ͬ���칹�庬��2�����ǻ���ȩ���������������ֲ�ͬ��ѧ��������ԭ�ӣ�Ҫ��ṹ���ܶԳƣ�����д���������ͬ���칹�壻
��6���Ա�����ϩΪԭ�Ͽɺϳɾ۱���ϩ������Ȼ�м���Ϊ����ϩ������д����ص�����ͼ��
�����㶨λ�� ����߿��л������ǰ����Ķ������ס���������ݶ����л���ѧ�Ļ���֪ʶ�������Ϸ��ӽṹ�еĹ����ţ�ץ���������ʣ��л����ӽṹʽ���л���ѧ��Ӧ����ʽ����ȷ��д��ͬ���칹�������л��ϳ����̵���ȷ��ʾ������Ҫ���������л���ѧ����֪ʶ��ȡ�����������Ӷ��ó����л�������Ľṹ���ٸ����л��ϳ�·�ش�������⡣

EΪ
 ��DΪ
��DΪ ��CΪ
��CΪ ��BΪ
��BΪ ��AΪ
��AΪ ��
����1�������ӷ����к����ǻ������������ԡ�������(�������ļ�)��������ˮ�ⷴӦ��1 mol������������ ��3 mol NaOH��Ӧ�������Ͽ��Է���������Ӧ�������Ϸ��ǻ��ڶ�λ������Է��������Ӧ��1mol��������Br2��Ӧ����������4 molBr2��
��2����������������������뷴Ӧ������Ũ������������
 ��ԭ��ˮ������1molNaOH�������зӻ�����������1molNaOH������ 2molNaOH������д����ػ�ѧ����ʽ��
��ԭ��ˮ������1molNaOH�������зӻ�����������1molNaOH������ 2molNaOH������д����ػ�ѧ����ʽ�����ǻ������ᣬ�������⣬����һ���Ȼ���һ���ǻ�������ĿҪ��ͬ���칹�庬��2�����ǻ���ȩ���������������ֲ�ͬ��ѧ��������ԭ�ӣ�Ҫ��ṹ���ܶԳƣ�����д���������ͬ���칹�壻
��6���Ա�����ϩΪԭ�Ͽɺϳɾ۱���ϩ������Ȼ�м���Ϊ����ϩ������д����ص�����ͼ��
�����㶨λ�� ����߿��л������ǰ����Ķ������ס���������ݶ����л���ѧ�Ļ���֪ʶ�������Ϸ��ӽṹ�еĹ����ţ�ץ���������ʣ��л����ӽṹʽ���л���ѧ��Ӧ����ʽ����ȷ��д��ͬ���칹�������л��ϳ����̵���ȷ��ʾ������Ҫ���������л���ѧ����֪ʶ��ȡ�����������Ӷ��ó����л�������Ľṹ���ٸ����л��ϳ�·�ش�������⡣

��ϰ��ϵ�д�
�����Ŀ

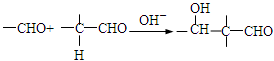
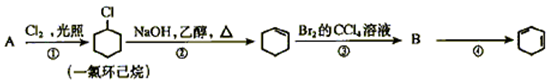
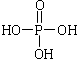


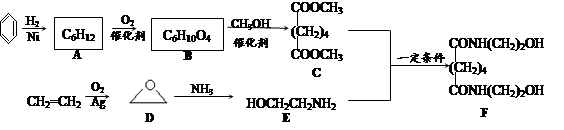
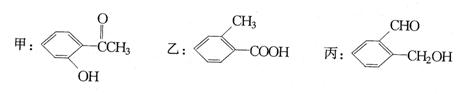


 ��
�� ��
�� D ��
D ��