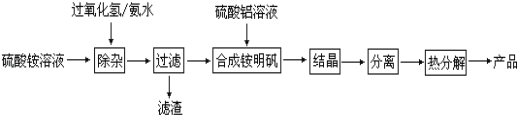
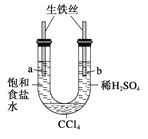
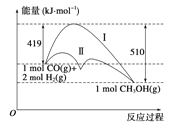
��Ŀ����
����Ŀ��һ�������£���ij�ܱ������м���һ������N2��H2�������淴Ӧ:N2(g)+3H2(g)![]() 2NH3(g) ��H = -92.2kJmol��1�����0��10���ڣ�c(H2)��С��0.75molL��1������˵����ȷ����( )
2NH3(g) ��H = -92.2kJmol��1�����0��10���ڣ�c(H2)��С��0.75molL��1������˵����ȷ����( )
A��10��15����c(NH3) ����������0.25mol L��1
B��10���ڰ�����ƽ����Ӧ����Ϊ0.025molL��1��s��1
C����ƽ����������NH3,v�� ����
D���÷�Ӧ���淴Ӧ�Ļ�ܲ�С��92.2kJmol��1
���𰸡�D
��������
���������A��0��10���ڣ�c(H2)��С��0.75molL��1��c(NH3)��Ũ��������0.5mol/L�����ŷ�Ӧ�Ľ��У���Ӧ���Ũ�ȼ�С����Ӧ���ʼ�С������10s��15s��c��NH3��������С��0.25mol/L��A�����B�����0��10s�ڣ�c��H2����С��0.75mol/L����c��NH3��������0.5mol/L������10s�ڰ�����ƽ����Ӧ����Ϊ��0.5mol/L��10s=0.05molL-1s-1��B�����C�������������Ũ�ȣ���Ӧ���ʼ�С�����ƽ����������NH3��ƽ��������Ӧ�����ƶ���v����С��C�����D����֪N2��g��+3H2��g��![]() 2NH3��g����H=-92.2kJmol-1����H=����Ӧ�Ļ��-�淴Ӧ�Ļ��=-92.2kJmol-1�����淴Ӧ�Ļ��=����Ӧ�Ļ��+92.2kJmol-1�������淴Ӧ�Ļ�ܲ�С��92.2kJmol-1��D����ȷ����ѡD��
2NH3��g����H=-92.2kJmol-1����H=����Ӧ�Ļ��-�淴Ӧ�Ļ��=-92.2kJmol-1�����淴Ӧ�Ļ��=����Ӧ�Ļ��+92.2kJmol-1�������淴Ӧ�Ļ�ܲ�С��92.2kJmol-1��D����ȷ����ѡD��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�