��Ŀ����
������Ԫ��X��Y��W��Ԫ�����ڱ��е����λ������ͼ��ʾ������ZΪ�ؿ��к�����ߵĽ���Ԫ�ء�����˵����ȷ����
|
|
|
|
|
| X |
Y |
| Z |
|
|
| W |
A����ҵ�ϵ��YW������Һұ������Y
B����̬�⻯����ȶ���:W>X
C�������Ӱ뾶�Ĵ�С˳��:r��Y+��>r��X-��
D��Y��W������������ˮ��������ܽ����Z
��ϰ��ϵ�д�
 С�����ϵ�д�
С�����ϵ�д�
�����Ŀ


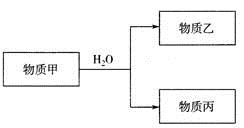
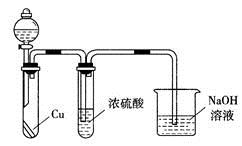

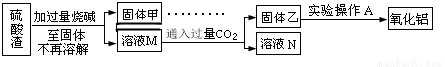
 ����C������E����G��ת��Ϊ��Ӧ���ȶ������������е�ʵ�����Ϊ________________��
����C������E����G��ת��Ϊ��Ӧ���ȶ������������е�ʵ�����Ϊ________________��