��Ŀ����
��֪ij�����£��ϳɰ���Ӧ���������£�
N2��g����3H2��g�� 2NH3��g��
2NH3��g��
��ʼŨ��/mol��L-1 1.0 3.0 0.2
2sĩŨ��/mol��L-1 0.6 1.8 1.0
4sĩŨ��/mol��L-1 0.4 1.2 1.4
���ð���Ũ�ȵ���������ʾ�÷�Ӧ������ʱ������˵���д�����ǣ� ��
N2��g����3H2��g��
 2NH3��g��
2NH3��g����ʼŨ��/mol��L-1 1.0 3.0 0.2
2sĩŨ��/mol��L-1 0.6 1.8 1.0
4sĩŨ��/mol��L-1 0.4 1.2 1.4
���ð���Ũ�ȵ���������ʾ�÷�Ӧ������ʱ������˵���д�����ǣ� ��
| A��2sĩ�����ķ�Ӧ���ʣ�0.4mol����L��s����1 |
| B��ǰ2sʱ���ڰ�����ƽ����Ӧ���ʣ�0.4mol����L��s����1 |
| C��ǰ4sʱ���ڰ�����ƽ����Ӧ���ʣ�0.3mol����L��s����1 |
| D��2s��4sʱ���ڰ�����ƽ����Ӧ���ʣ�0.2mol����L��s����1 |
A
A�����ƽ����Ӧ���ʵĶ��壬2sĩ��������˲ʱ���ʣ��ڱ����������Dz������ģ�������0��2sʱ���ڣ�Ũ�ȵ�����ֵΪ1.0mol/L��0.2mol/L��0.8mol/L����ǰ2s������ƽ����Ӧ���ʣ�0.8mol/L��2s��0.4mol/��L��s����B����ȷ��ǰ4s�ڰ�����ƽ����Ӧ���ʣ���1.4��0.2��mol/L��4s��0.3mol/��L��s������C����ȷ��2s��4sʱ���ڰ�����ƽ����Ӧ���ʣ���1.4��1.0��mol/L�£�4��2��s��0.2mol/��L��s������D����ȷ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 2C(g)��D(g)��2 min B��Ũ�ȼ���0.6 mol��L��1���Դ˷�Ӧ���ʵı�ʾ��ȷ���ǣ� ��
2C(g)��D(g)��2 min B��Ũ�ȼ���0.6 mol��L��1���Դ˷�Ӧ���ʵı�ʾ��ȷ���ǣ� �� 2NH3(g)����H��0�������о�Ŀ�ĺ�ʾ��ͼ������� (����)��
2NH3(g)����H��0�������о�Ŀ�ĺ�ʾ��ͼ������� (����)��
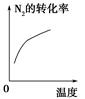
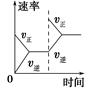
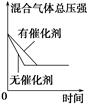
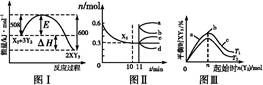
 C��g��������һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ����������������˵����ȷ����
C��g��������һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ����������������˵����ȷ���� 2XY3(g)��ͼ���ʾ��һ���¶��´˷�Ӧ�����е������仯;ͼ���ʾ��2 L���ܱ������з�ӦʱX2�����ʵ�����ʱ��ı仯����;ͼ���ʾ��������������������,�ı���ʼ��Y2�����ʵ����Դ˷�Ӧƽ���Ӱ�졣
2XY3(g)��ͼ���ʾ��һ���¶��´˷�Ӧ�����е������仯;ͼ���ʾ��2 L���ܱ������з�ӦʱX2�����ʵ�����ʱ��ı仯����;ͼ���ʾ��������������������,�ı���ʼ��Y2�����ʵ����Դ˷�Ӧƽ���Ӱ�졣