��Ŀ����
�±���25��CʱijЩ�ε�Ũ�Ȼ�����������ĵ���ƽ�ⳣ��������˵����ȷ���ǣ� ��
|
��ѧʽ |
AgCl |
Ag2CrO4 |
CH3COOH |
HClO |
H2CO3 |
|
KSP��Ka |
KSP=1.8��10-10 |
KSP=2.0��10-12 |
Ka=1.8��10-5 |
Ka=3.0��10-8 |
Ka1=4.1��10-7 Ka2=5.6��10-11 |
A����ͬŨ��CH3COONa��NaClO�Ļ��Һ�У�������Ũ�ȵĴ�С��ϵ��
c��Na+��>c��ClO-��>c��CH3COO-��>c��OH-��>c��H+��
B��̼������Һ�еμ�������ˮ�����ӷ���ʽΪCO32-+Cl2=HCO3-+Cl-+HClO
C����0.1mol��L-1CH3COOH��Һ�еμ�NaOH��Һ��c��CH3COOH����c��CH3COO-��=5��9����ʱ��ҺpH=5
D����Ũ�Ⱦ�Ϊ1��10-3mol��L-1��KCl��K2CrO4���Һ�еμ�1��10-3mol��L-1��AgNO3��Һ��CrO42-���γɳ���
C
��������
������������ݵ���ƽ�ⳣ����֪����ǿ��˳��Ϊ��CH3COOH��HClO������Խ������Ӧ�������������ˮ��̶�Խ����Һ��pHԽ��������ͬŨ��CH3COONa��NaClO�Ļ��Һ�У�������Ũ�ȵĴ�С��ϵ��c��Na+���� c��CH3COO-����c��ClO-���� c��OH-����c��H+������A����̼�������ǿ�ڴ�����ģ��������������ǿ��HCO3��������̼������Һ�еμ�������ˮ�����ӷ���ʽΪH2O��CO32-+Cl2��HCO3-+Cl-+HClO������B����ȷ��ѡ��C�и��ݵ���ƽ�ⳣ����֪��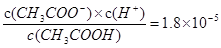 ������c��CH3COOH����c��CH3COO-����5��9�����������ӵ�Ũ����10��5mol/L����pH��5����C��ȷ������Һ�������Ӻ�CrO42��Ũ�Ⱦ�Ϊ0.001mol/Lʱ��Ҫ������Ӧ�ij���������Ҫ�����ӵ�Ũ�ȷֱ���
������c��CH3COOH����c��CH3COO-����5��9�����������ӵ�Ũ����10��5mol/L����pH��5����C��ȷ������Һ�������Ӻ�CrO42��Ũ�Ⱦ�Ϊ0.001mol/Lʱ��Ҫ������Ӧ�ij���������Ҫ�����ӵ�Ũ�ȷֱ���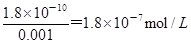 ��
�� 4.47��10��5mol/L���������������ij������Ȼ�����ѡ��D����ȷ����ѡC��
4.47��10��5mol/L���������������ij������Ȼ�����ѡ��D����ȷ����ѡC��
���㣺����������ʵĵ���ƽ�⡢�ܶȻ�������Ӧ��
�������������е��Ѷȵ����⣬�����ۺ���ǿ�������߿��������ڵ���ѧ����ѧϰ��Ȥ������ѧ����ѧϰ�����ԡ��ڽ���漰����Ũ�ȴ�С�Ƚ�ʱҪע����������Ե���ƽ���Ӱ�죬���õ���غ㡢�����غ��������Ŀ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���2012?���ģ�⣩�±���25��CʱijЩ����ĵ���ƽ�ⳣ��������˵����ȷ���ǣ�������
|
��2012?�㽭ģ�⣩�±���25��CʱijЩ�ε�Ũ�Ȼ�����������ĵ���ƽ�ⳣ��������˵����ȷ���ǣ�������
|
�±���25��CʱijЩ�ε�Ũ�Ȼ�����������ĵ���ƽ�ⳣ��������˵����ȷ���ǣ� ��
| ��ѧʽ | AgCl | Ag2CrO4 | CH3COOH | HClO | H2CO3 |
| KSP��Ka | KSP=1.8��10-10 | KSP=2.0��10-12 | Ka=1.8��10-5 | Ka=3.0��10-8 | Ka1=4.1��10-7 Ka2=5.6��10-11 |
c��Na+��>c��ClO-��>c��CH3COO-��>c��OH-��>c��H+��
B��̼������Һ�еμ�������ˮ�����ӷ���ʽΪCO32-+Cl2=HCO3-+Cl-+HClO
C����0.1mol��L-1CH3COOH��Һ�еμ�NaOH��Һ��c��CH3COOH����c��CH3COO-��=5��9����ʱ��ҺpH=5
D����Ũ�Ⱦ�Ϊ1��10-3mol��L-1��KCl��K2CrO4���Һ�еμ�1��10-3mol��L-1��AgNO3��Һ��CrO42-���γɳ���
�±���25��CʱijЩ�ε�Ũ�Ȼ�����������ĵ���ƽ�ⳣ��������˵����ȷ���ǣ� ��
|
��ѧʽ |
CH3COOH |
HClO |
H2CO3 |
|
Ka |
Ka=1.8��10-5 |
Ka=3.0��10-8 |
Ka1=4.1��10-7 Ka2=5.6��10-11 |
A�������ʵ���Ũ�ȵ�NaClO��NaHCO3 �����Һ�� c(HClO)��c(ClO��)��c(HCO3��)��c(CO32��)��c(H2CO3)
B�������������Һ��ͨ������������̼��������ӷ���ʽΪClO-+CO2+H2O=CO32-+2HClO
C��25��ʱ�����ȵ���0.1mol/L�Ĵ�������Һ�У���Һ�ʺ�ɫ
D�����H+��������CH3COO-��ClO-��CO32-
�±���25��CʱijЩ�ε�Ũ�Ȼ�����������ĵ���ƽ�ⳣ��������˵����ȷ����
|
��ѧʽ |
AgCl |
Ag2CrO4 |
CH3COOH |
HClO |
H2CO3 |
|
KSP��Ka |
KSP=1.8��10-10 |
KSP=2.0��10-12 |
Ka=1.8��10-5 |
Ka=3.0��10-8 |
Ka1=4.1��10-7 Ka2=5.6��10-11 |
A����ͬŨ��CH3COONa��NaClO�Ļ��Һ�У�������Ũ�ȵĴ�С��ϵ��
c��Na+��>c��ClO-��>c��CH3COO-��>c��OH-��>c��H+��
B��̼������Һ�еμ�������ˮ�����ӷ���ʽΪCO32-+Cl2=HCO3-+Cl-+HClO
C����0.1mol��L-1CH3COOH��Һ�еμ�NaOH��Һ��c��CH3COOH����c��CH3COO-��=5��9����ʱ��ҺpH=5
D����Ũ�Ⱦ�Ϊ1��10-3mol��L-1��KCl��K2CrO4���Һ�еμ�1��10-3mol��L-1��AgNO3��Һ��CrO42-���γɳ���