��Ŀ����
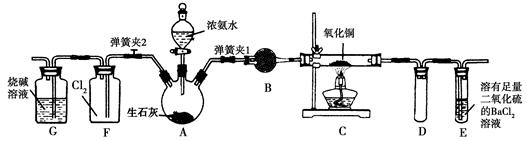
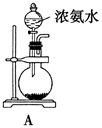
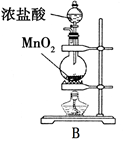
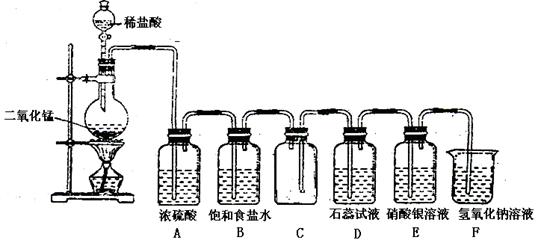
ij�о���ѧϰС�鰴��ͼ��ʾװ�ý���̽��ʵ�顣
��ش��������⣺
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2��ʵ������У�װ��B������������ ��������Ӧ�����ӷ���ʽΪ ��
��3��װ��C�з����������� ����˵��SO2���е������� ��
��4��װ��D��Ŀ����̽��SO2��Ʒ�����õ��ȶ��ԣ���д��ʵ�����������
��
��5�������ʵ����֤װ��B��Һ�Ƿ���SO42�� ��
��6�����ڿ�ͼ�ڻ���SO2β������װ��ͼ��
��1�� Na2SO3+H2SO4��Ũ����Na2SO4+SO2��+H2O ��2�֣�
��2����Һ���Ϻ�ɫ��1�֣���Ϊ��ɫ��1�֣����Ϻ�ɫ���Ը��������ɫ
5SO2+2MnO4��+2H2O��5SO42��+2Mn2++4H+��2�֣�
��3����ɫ��Һ���ֻ�ɫ��1�֣����ǻ���� ��1�֣� �����ԣ�1�֣�
��4��Ʒ����Һ��ɫ�رշ�Һ©��������1�֣�����ȼ�ƾ��Ƽ��ȣ�1�֣�����Һ�ָ���ɫ��1�֣�
��ȡ������ɫ��Ʒ����Һ���Թܣ�1�֣������ȣ�1�֣�����Һ�ָ���ɫ��1�֣�
��5��ȡ��������Һ���μ��������Ȼ�����Һ�����а�ɫ����������˵������SO42����������������ȫ�Ե�1�֣������ް�ɫ����������˵��û��SO42����������ȫ�Ե�1�֣��������ȼ�������ϡ�����ữ�������۷֣�
��6�� ��2�֣�����װ��1�֣���ע�Լ�1�֣�װ�á��Լ�
��2�֣�����װ��1�֣���ע�Լ�1�֣�װ�á��Լ�
���������������1��Ũ������ǿ�ᣬ���������Ʒ�Ӧ���������ơ�SO2��ˮ�����װ��A���Ʊ�SO2�ģ�����װ��A�з�����Ӧ�Ļ�ѧ����ʽΪNa2SO3+H2SO4��Ũ����Na2SO4+SO2��+H2O��
��2��SO2���л�ԭ�ԣ����Ը��������Һ����ǿ�����ԣ���SO2����������ԭ��Ӧ�����ʵ������У�װ��B��������������Һ���Ϻ�ɫ��Ϊ��ɫ���Ϻ�ɫ���Ը��������ɫ��������Ӧ�����ӷ���ʽΪ5SO2+2MnO4��+2H2O��5SO42��+2Mn2++4H+��
��3��SO2��SԪ�صĻ��ϼ��ǣ�4�ۣ����������ԡ�Na2S��SԪ�صĻ��ϼ��ǣ�2�ۣ����л�ԭ�ԡ����SO2ͨ�뵽Na2S��Һ�з���������ԭ��Ӧ���ɵ���S����������װ��C�з�������������ɫ��Һ���ֻ�ɫ���ǻ��������Ӧ�����ӷ���ʽΪSO2��2S2����2H2O��3S����4OH����
��4������SO2��Ư���Բ��ȶ����ڼ��ȵ��������ָֻ���ԭ������ɫ�����̽��SO2��Ʒ�����õ��ȶ��Ե�ʵ�������������Ʒ����Һ��ɫ�رշ�Һ©����������ȼ�ƾ��Ƽ��ȣ���Һ�ָ���ɫ��ȡ������ɫ��Ʒ����Һ���Թܣ����ȣ���Һ�ָ���ɫ��
��5�����ᱵ�Dz�����ˮҲ��������İ�ɫ�������ݴ˿��Լ���SO42���������֤װ��B��Һ�Ƿ���SO42���ķ�����ȡ��������Һ���μ��������Ȼ�����Һ�����а�ɫ����������˵������SO42�������ް�ɫ����������˵��û��SO42����
��6��SO2�Ǵ�����Ⱦ���Ҫβ������������SO2���������������������������Һ���գ������ȷ��װ��ͼΪ ��
��
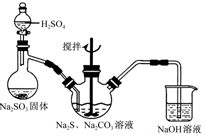
���㣺����SO2�Ʊ���������֤��β�������Լ����Ӽ���

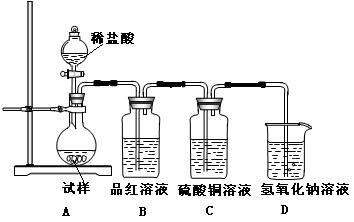
��ʵ�����п�����ͼ��ʾװ����ȡ����ء��������ƺ�̽����ˮ�����ʡ�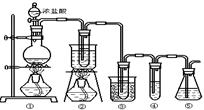
ͼ�У���Ϊ��������װ�ã��ڵ��Թ���ʢ��15mL30��KOH��Һ����������ˮԡ�У��۵��Թ���ʢ��15mL 8%NaOH��Һ�������ڱ�ˮԡ�У��ܵ��Թ��������ɫʯ����Һ����Ϊβ������װ�á�
����д���пհף�
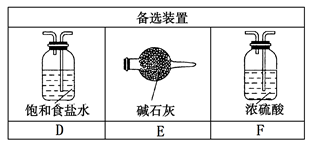
��1����ȡ����ʱ������ƿ�����һ�����Ķ������̣�ͨ��______����д�������ƣ�����ƿ�м���������Ũ���ᡣʵ��ʱΪ�˳�ȥ�����е��Ȼ������壬���ڢ����֮�䰲װʢ��_________����д���б����ĸ���ľ���װ�á�
| A����ʯ�� | B������ʳ��ˮ | C��Ũ���� | D������̼��������Һ |
��3����ʵ������ȡ�������Ƶ����ӷ���ʽ�ǣ� ��
��4��ʵ���пɹ۲쵽�ܵ��Թ�����Һ����ɫ���������±仯������д�±��еĿհף�
| ʵ������ | ԭ�� |
| ��Һ�������ɫ��Ϊ___ɫ | ������ˮ��Ӧ���ɵ�H+ʹʯ���ɫ |
| �����Һ��Ϊ��ɫ | ______________________________________ |
| Ȼ����Һ����ɫ��Ϊ___ɫ | _________________________________________ |
����֪��Ũ���������������Һ�У���������ɫ���壬����Һ���Ϻ�ɫ��ȥ������һ��������ԭ��Ӧ����ϵ�й���KCl��Cl2��H2SO4��H2O��KMnO4��MnSO4��K2SO4�������ʣ�
��1���÷�Ӧ�У����ϼ����ߵķ�Ӧ������������������
��2��д��һ�����������������ʵ�������ԭ��Ӧ����ʽ������������������������
��3��������Ӧ�У�����������������1 mol�������ڷ�Ӧ�еõ� ������mol���ӡ�
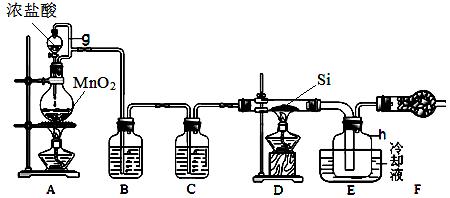
��ij�о���ѧϰС����������װ���Ʊ�Ư�ۣ�������Ư����Ч�ɷֵ����������ⶨ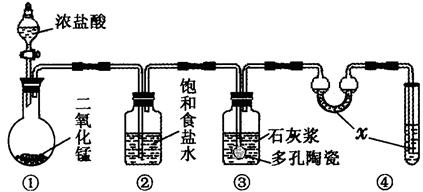
��1��װ�â��е�x�Լ�Ϊ ��
��2��װ�â��з�����Ӧ�Ļ�ѧ����ʽΪ ���÷�Ӧ�Ƿ��ȷ�Ӧ����Ӧ�¶Ƚϸ�ʱ�и���Ӧ�������Ľ���ʵ��װ���Լ��ٸ���Ӧ�����ķ�����
_________________________��
��3���ⶨƯ����Ч�ɷֵ���������
��ȡ1.000 gƯ������ƿ�У���ˮ�ܽ⣬������Һ��pH���Ե���Ϊָʾ������0.1000 mol��L��1 KI��Һ���еζ�����Һ�����ȶ�dz��ɫʱΪ�ζ��յ㡣��Ӧԭ��Ϊ��
3ClO��+ I�� = 3Cl��+ IO3�� IO3�� + 5I�� + 3H2O = 6OH�� + 3I2
ʵ�����������±���ʾ��
| ����� | 1 | 2 | 3 |
| KI��Һ���/mL | 19.98 | 20.02 | 20.00 |
��Ư������Ч�ɷֵ���������Ϊ �����ζ�������δ�������Һ�ֲ���dz��ɫʱ��ֹͣ�ζ�����ⶨ����� ���ƫ�ߡ�����ƫ�͡�����Ӱ�족��