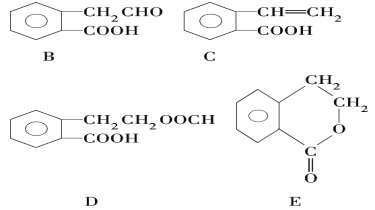
��Ŀ����
����Ŀ����NaOH��Һ���������е�SO2�������õ�Na2SO3��Һ���е�⣬��ѭ������NaOH��ͬʱ�õ�H2SO4����ԭ����ͼ��ʾ(�缫����Ϊʯī)
ͼ1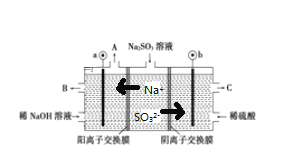 ͼ2
ͼ2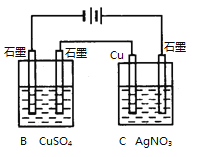
(1)ͼ1��a��Ҫ���ӵ�Դ��__________(����������������)����SO32-�ŵ�ĵ缫��Ӧ__________
(2)��ͼ2��ʾ��װ���У���ͨ��ֱ����5 minʱ��ͭ�缫��������2.16g���Իش�
����ҺpH�仯��B__________��C__________(��������������С������������)��
��ͨ��5 minʱ��B�й��ռ�224mL����(��״��)����Һ���Ϊ200mL����ͨ��ǰCuSO4��Һ�����ʵ���Ũ��Ϊ__________(����ǰ����Һ����ޱ仯)��
���𰸡����� SO32--2e-+H2O=SO42-+2H+ ��С ���� 0.025 mol��L-1
��������
(1)���ݵ������Һ���������ӵ��ƶ������жϵ缫������������������aΪ������bΪ������![]() ������ʧȥ���ӱ��
������ʧȥ���ӱ��![]() �����ܰ������������ӷŵ���������������C��������������H2SO4���������ŵ�����Ϊ�����������������ݴ˷������
�����ܰ������������ӷŵ���������������C��������������H2SO4���������ŵ�����Ϊ�����������������ݴ˷������
(2)��ͼ������Ϣ��֪��B��C����װ�ô�����ͬһ��·�С�����ͭ�缫��������2.16 g�������ж�ͭ�缫Ϊ���������Դ��X�缫Ϊ������Y�缫Ϊ���������ԣ�B�ǵ��ء�C�ǵ��װ�ã��ݴ˷���������⡣
(1)������������֪��a�ӵ�Դ������![]() ����������Ӧ���ŵ�ĵ缫��ӦʽΪ��
����������Ӧ���ŵ�ĵ缫��ӦʽΪ��![]() ���ʴ�Ϊ��������
���ʴ�Ϊ��������![]() ��
��
(2)�ٸ�������������֪��B�е������ͭ��Һ�������ᣬ��Һ��������Ũ������pH��С��C�ǵ��װ�ã�����������ӦΪAg++e-�TAg��������ӦΪAg-e-�TAg+����ҺŨ�Ȳ��䣬��pH���䣬�ʴ�Ϊ����С�����䣻
��ͨ��5 min��·��ͨ���ĵ���Ϊn(e-)=n(Ag)=![]() ��B���������ռ���O2��n(O2)=
��B���������ռ���O2��n(O2)=![]() ����ΪB�й��ռ�����״����224 mL���壨0.01mol��������B������һ������������0.005mol���ɴ˸��ݵ���ת���غ㣬����ȷ��B��һ������ͭ0.005mol��������Һ���Ϊ200 mL�����Լ����ͨ��ǰCuSO4��Һ�����ʵ���Ũ��Ϊ
����ΪB�й��ռ�����״����224 mL���壨0.01mol��������B������һ������������0.005mol���ɴ˸��ݵ���ת���غ㣬����ȷ��B��һ������ͭ0.005mol��������Һ���Ϊ200 mL�����Լ����ͨ��ǰCuSO4��Һ�����ʵ���Ũ��Ϊ![]() 0.025 mol��L��1���ʴ�Ϊ��0.025 mol��L��1��
0.025 mol��L��1���ʴ�Ϊ��0.025 mol��L��1��