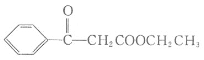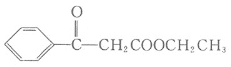МвДҝДЪИЭ
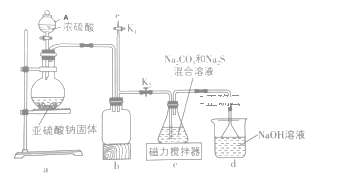
ЎҫМвДҝЎҝДі·ПҫЙөзіШІДБПөДЦчТӘіЙ·ЦОӘоЬЛбп®ЈЁLiCoO2Ј©Ј¬»№ә¬УРТ»¶ЁБҝөДМъЎўВБЎўНӯөИФӘЛШөД»ҜәПОпЈ¬Жд»ШКХ№ӨТХИзНјЛщКҫЈ¬ЧоЦХҝЙөГөҪCo2O3әНп®СОЎЈ
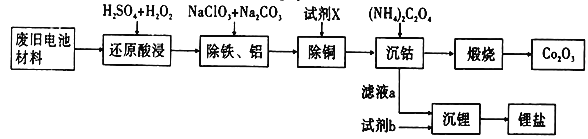
ТСЦӘЈәCoC2O4ЎӨ2H2OОўИЬУЪЛ®Ј¬ЛьөДИЬҪв¶ИЛжОВ¶ИЙэёЯ¶шЦрҪҘФцҙуЈ¬ЗТДЬУл№эБҝөДC2O42ЈӯАлЧУЙъіЙCoЈЁC2O4Ј©n2ЈЁnЈӯ1Ј©Јӯ¶шИЬҪвЎЈ
ЈЁ1Ј©Ў°»№ФӯЛбҪюЎұ№эіМЦРоЬЛбп®·ҙУҰөДАлЧУ·ҪіМКҪ________________________________________Ј»ОВ¶ИНЁіЈҝШЦЖФЪ40ЎжТФПВөДФӯТтКЗ_______________________________________________ЎЈ
ЈЁ2Ј©Ў°іэВБМъЎұ№эіМөДБҪЦЦКФјБөДЧчУГ·ЦұрКЗ____________________Ј¬______________________ЎЈ
ЈЁ3Ј©Ў°іэНӯЎұЛщУГКФјБXОӘH2SЈ¬КФРҙіцёГ·ҙУҰөДАлЧУ·ҪіМКҪ______________________________ЎЈ
ЈЁ4Ј©Ў°іБоЬЎұ№эіМЦРЈ¬ЈЁNH4Ј©2C2O4өДјУИлБҝЈЁНјaЈ©ЎўіБөн·ҙУҰөДОВ¶ИЈЁНјbЈ©УлоЬөДіБөнВК№ШПөИзНјЛщКҫЈә
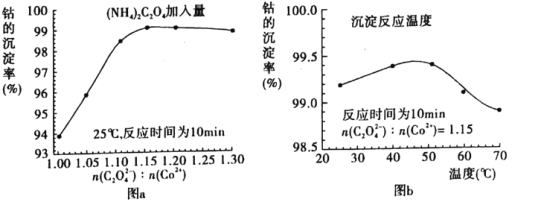
ўЩЛжnЈЁC2O42ЈӯЈ©ЈәNЈЁCo2Ј«Ј©ұИЦөөДФцјУЈ¬оЬөДіБөнВКПИЦрҪҘФцҙуәуУЦЦрҪҘјхРЎөДФӯТт________________________________________________________________________ЎЈ
ўЪіБөн·ҙУҰКұјдОӘ10 minЈ¬ОВ¶ИФЪ50ЎжТФЙПКұЈ¬ЛжОВ¶ИЙэёЯ¶шоЬөДіБөнВКПВҪөөДҝЙДЬФӯТтКЗ_________________________________________________________ЎЈ
Ўҫҙр°ёЎҝ2LiCoO2 + H2O2 + 6H+ = 2Li+ + 2Co2+ + O2Ўь+ 4H2O ·АЦ№H2O2КЬИИ·ЦҪв Ҫ«Fe2+Сх»ҜОӘFe3+ өчҪЪpHЈ¬ҙЩҪшЛ®ҪвіэИҘFe3+әНAl3+ Cu2+ + H2S = CuSЎэ+2H+ №эБҝөДC2O42-УлCo2+·ҙУҰЙъіЙCo(C2O4)n2(n-1)-¶шИЬҪв ЛьөДИЬҪв¶ИЛжОВ¶ИЙэёЯ¶шЦрҪҘФцҙу
ЎҫҪвОцЎҝ
ЈЁ1Ј©Ў°»№ФӯЛбҪюЎұ№эіМЦР+3јЫCoұ»H2O2»№ФӯОӘCo2+Ј»H2O2ФЪёЯОВМхјюПВТЧ·ЦҪвЈ»
(2) NaClO°СFe2+Сх»ҜОӘFe3+Ј¬Na2CO3ҝЙТФөчҪЪpHЈ¬ҙЩҪшFe3+ЎўAl3+Л®ҪвЙъіЙЗвСх»ҜОпіБөнЈ»
ЈЁ3Ј©Cu2+УлH2S·ҙУҰЙъіЙCuSіБөнЎЈ
ЈЁ4Ј©ўЩCoC2O4ЎӨ2H2OДЬУл№эБҝөДC2O42ЈӯАлЧУЙъіЙCoЈЁC2O4Ј©n2ЈЁnЈӯ1Ј©Јӯ¶шИЬҪвЈ»ўЪCoC2O4ЎӨ2H2OОўИЬУЪЛ®Ј¬ИЬҪв¶ИЛжОВ¶ИЙэёЯ¶шЦрҪҘФцҙуЎЈ
ЈЁ1Ј©Ў°»№ФӯЛбҪюЎұ№эіМЦР+3јЫCoұ»H2O2»№ФӯОӘCo2+Ј¬·ҙУҰөДАлЧУ·ҪіМКҪКЗ2LiCoO2 + H2O2 + 6H+ = 2Li+ + 2Co2+ + O2Ўь+ 4H2OЈ»H2O2ФЪёЯОВМхјюПВТЧ·ЦҪвЈ¬ОВ¶ИНЁіЈҝШЦЖФЪ40ЎжТФПВҝЙТФ·АЦ№H2O2КЬИИ·ЦҪвЈ»
(2) NaClO°СFe2+Сх»ҜОӘFe3+Ј¬Na2CO3ҝЙТФөчҪЪpHЈ¬ҙЩҪшFe3+ЎўAl3+Л®ҪвЙъіЙЗвСх»ҜОпіБөнЈ»
ЈЁ3Ј©Cu2+УлH2S·ҙУҰЙъіЙCuSіБөнЈ¬·ҙУҰөДАлЧУ·ҪіМКҪКЗCu2+ + H2S = CuSЎэ+2H+ЎЈ
ЈЁ4Ј©ўЩCoC2O4ЎӨ2H2OДЬУл№эБҝөДC2O42ЈӯАлЧУЙъіЙCoЈЁC2O4Ј©n2ЈЁnЈӯ1Ј©Јӯ¶шИЬҪвЈ¬ЛщТФЛжnЈЁC2O42ЈӯЈ©ЈәNЈЁCo2Ј«Ј©ұИЦөөДФцјУЈ¬оЬөДіБөнВКПИЦрҪҘФцҙуәуУЦЦрҪҘјхРЎЈ»
ўЪCoC2O4ЎӨ2H2OОўИЬУЪЛ®Ј¬ИЬҪв¶ИЛжОВ¶ИЙэёЯ¶шЦрҪҘФцҙуЈ¬ЛщТФОВ¶ИФЪ50ЎжТФЙПКұЈ¬ЛжОВ¶ИЙэёЯ¶шоЬөДіБөнВКПВҪөЎЈ

ЎҫМвДҝЎҝҪсУРКТОВПВЛДЦЦИЬТәЈ¬УР№ШРрКцІ»ХэИ·өДКЗ(ЎЎЎЎ)
ўЩ | ўЪ | ўЫ | ўЬ | |
ЕЁ¶Иc/mol/L | 0.1 | 0.1 | 0.1 | 0.1 |
ИЬТә | °ұЛ® | CH3COONaИЬТә | ҙЧЛб | СОЛб |
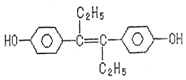
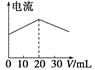
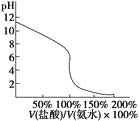
A.ФЪ20 mL ўЩИЬТәЦРЦрөОјУИлўЫИЬТәЈ¬ИЬТәөјөзДЬБҰұд»ҜИзПВНј
B.ўЪЎўўЫБҪИЬТәөИМе»э»мәПЈ¬АлЧУЕЁ¶ИЈә2c(Na+)ЈҪc(CH3COO-)+c(CH3COOH)
C.ўЩЎўўЬБҪИЬТәөИМе»э»мәПЈ¬АлЧУЕЁ¶ИЈәc(Cl-)>c(NH4Ј«)>c(H+)>c(OH-)
D.УГўЬөО¶ЁўЩЈ¬өО¶ЁЗъПЯИзПВНјЈ¬ҝЙУГ·УМӘЧчЦёКҫјБ
ЎҫМвДҝЎҝДіКөСйРЎЧйУГ0.50 mol/L NaOHИЬТәәН0.50 mol/LБтЛбИЬТәҪшРРЦРәНИИөДІв¶ЁЎЈ
ўсЈ®ЕдЦЖ0.50 mol/L NaOHИЬТә
ЈЁ1Ј©ИфКөСйЦРҙуФјТӘК№УГ245 mL NaOHИЬТәЈ¬ЦБЙЩРиТӘіЖБҝNaOH№ММе________gЎЈ
ЈЁ2Ј©ЕдЦЖ№эіМЦРРиТӘУГөҪөДІЈБ§ТЗЖчіэЙХұӯЎўІЈБ§°фНв»№РиТӘУР_________Ўў___________ЎЈ
ўтЈ®Ів¶ЁЦРәНИИөДКөСйЧ°ЦГИзПВНјЛщКҫЎЈ
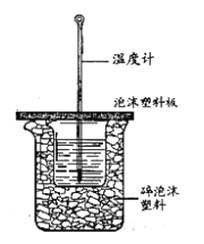
ЈЁ3Ј©ҙуРЎЙХұӯЦ®јдМоВъЛйЕЭДӯЛЬБПөДЧчУГКЗ________Ј¬ҙУКөСйЧ°ЦГЙПҝҙЈ¬НјЦРИұЙЩөДТ»ЦЦІЈБ§ТЗЖч________ЎЈ
ЈЁ4Ј©К№УГІ№И«ТЗЖчәуөДЧ°ЦГҪшРРКөСйЈ¬ИЎ50mL 0.25molЈҜL H2SO4ИЬТәУл50mL0.55 molЈҜL NaOHИЬТәФЪРЎЙХұӯЦРҪшРРЦРәН·ҙУҰЈ¬КөСйКэҫЭИзПВұнЎЈ
ўЩЗлМоРҙПВұнЦРөДҝХ°ЧЈә
КөСйҙОКэ | ЖрКјОВ¶Иt1/Ўж | ЦХЦ№ОВ¶И | ОВ¶ИІоЖҪҫщЦө | ||
H2SO4 | NaOH | ЖҪҫщЦө | |||
1 | 26.2 | 26.0 | 26.1 | 29.5 | _____Ўж |
2 | 27.0 | 27.4 | 27.2 | 33.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.2 | |
4 | 26.4 | 26.2 | 26.3 | 29.8 | |
ўЪНЁ№эјЖЛгҝЙөГЦРәНИИЎчHЈҪ___________(ҫ«И·өҪРЎКэөгәуТ»О»)
ўЫЙПКцКөСйКэЦөҪб№ыУл57.3 kJ/molУРЖ«ІоЈ¬ІъЙъЖ«ІоөДФӯТтҝЙДЬКЗ____ЎЈЈЁМоЧЦДёЈ©
AЈ®КөСйЧ°ЦГұЈОВЎўёфИИР§№ыІо
BЈ®БҝИЎNaOHИЬТәөДМе»эКұСцКУ¶БКэ
CЈ®·Ц¶аҙО°СNaOHИЬТәө№ИлКўУРБтЛбөДРЎЙХұӯЦР
DЈ®УГОВ¶ИјЖІв¶ЁNaOHИЬТәЖрКјОВ¶ИәуЦұҪУІв¶ЁH2SO4ИЬТәөДОВ¶И
ЈЁ5Ј©КөСйЦРИфУГ60mL0Ј®25molЎӨLЈӯ1H2SO4ИЬТәёъ50mL0Ј®55molЎӨLЈӯ1NaOHИЬТәҪшРР·ҙУҰЈ¬УлЙПКцКөСйПаұИЈ¬Лщ·ЕіцөДИИБҝ_____________ЈЁМоЎ°ПаөИЎұЎўЎ°І»ПаөИЎұЈ©Ј¬ЛщЗуЦРәНИИ___________ЈЁМоЎ°ПаөИЎұЎўЎ°І»ПаөИЎұЈ©Ј»ИфУГ50mL0.50molЎӨLЈӯ1ҙЧЛбҙъМжH2SO4ИЬТәҪшРРЙПКцКөСйЈ¬ІвөГ·ҙУҰЗ°әуОВ¶ИөДұд»ҜЦө»б_________ЈЁМоЎ°Ж«ҙуЎұЎўЎ°Ж«РЎЎұЎўЎ°І»КЬУ°ПмЎұЈ©ЎЈ