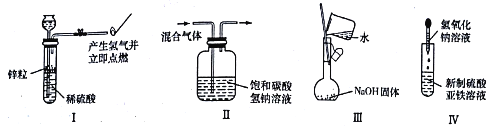
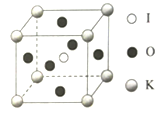
��Ŀ����
����Ŀ�������ܱ������������淴Ӧ��Z������+W������![]() X��g��+Y����������H����t1ʱ�̷�Ӧ�ﵽƽ�⣬��t2ʱ����С���������t3ʱ���ٴδﵽƽ��״̬���ٸı������������й�˵������ȷ����
X��g��+Y����������H����t1ʱ�̷�Ӧ�ﵽƽ�⣬��t2ʱ����С���������t3ʱ���ٴδﵽƽ��״̬���ٸı������������й�˵������ȷ����

A. Z��W�ڸ���������һ�ֿ���Ϊ��̬
B. t1~t2ʱ�����t3ʱ�̺���ʱ��η�Ӧ��ϵ�������ƽ��Ħ��������������ȡ�
C. ���÷�Ӧֻ��ij�¶�T0�����Է����У���÷�Ӧ��ƽ�ⳣ��K���¶����߶�����
D. ���ڸ��¶��´˷�Ӧƽ�ⳣ������ʽΪK=c��X������t1~t2ʱ�����t3ʱ�̺��XŨ�Ȳ����
���𰸡�C
������������ͼ���֪������Ӧ���ʲ��淴Ӧʱ���ѹǿ�ĸı���ı�������Z��W���������壬A���������ͼ���֪��X�����壬Y���ܲ���������������壬��Ӧ�����������Ħ������ʼ�ղ������ͬ������t1~t2ʱ�����t3ʱ�̺�ʱ��η�Ӧ��ϵ�������ƽ��Ħ�������������Ҳ���ܲ��ȣ�B��������Ϊ�÷�Ӧֻ��ij�¶�T0�����Է����У�����H-TS<0���ó��÷�Ӧ�����ȷ�Ӧ�������¶�ƽ�����ƣ�ƽ�ⳣ��K������C��ȷ��ƽ�ⳣ��ֻ���¶��й������¶��ºⳣ������ʽΪK=c��X���Ƕ�ֵ������t1~t2ʱ�����t3ʱ�̺��XŨ����ȣ�D��������ȷѡ��C��

 �㽭��У��ʦ���ϵ�д�
�㽭��У��ʦ���ϵ�д� ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д�