��Ŀ����
����Ŀ����.������Mg5Al3��OH��19��H2O��4������������ȼ���ϣ�����ʱ�����»�ѧ����ʽ�ֽ⣺2Mg5Al3��OH��19��H2O��4 ![]() 27H2O��+10MgO+3Al2O3
27H2O��+10MgO+3Al2O3
���Բ���A��������Ԫ����ɵĻ����ij�о�С�鰴��ͼ����̽������ɣ�
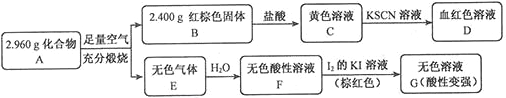
��1��A�����Ԫ��Ϊ_________����Ԫ�ط��ű�ʾ������ѧʽΪ______��
��2����ҺC���ܽ�ͭƬ���оٸ÷�Ӧ��һ��ʵ��Ӧ��____________��
��3����֪������A����ϡ���ᷴӦ������һ�ֵ���ɫ�������һ�����壨����µ��ܶ�Ϊ1.518 g��L-1������������ӵĵ���ʽΪ____��д���÷�Ӧ�����ӷ���ʽ__________��
��4��д��F��G��Ӧ�Ļ�ѧ����ʽ_____________�����ʵ�鷽��̽����ҺG�е���Ҫ����������H2O��H+��K+��I-��______________��
���𰸡� S��Fe Fe3S4 ��ӡˢ��·�� ![]() Fe3S4+6H+ =3H2S��+3Fe2+ +S H2SO3+I2+H2O =H2SO4+2HI ȡ��ҺG���������BaCl2��Һ����������ɫ����������SO42�����˺�ȡ��Һ���μ�H2O2��Һ�����ٲ�����ɫ����������H2SO3
Fe3S4+6H+ =3H2S��+3Fe2+ +S H2SO3+I2+H2O =H2SO4+2HI ȡ��ҺG���������BaCl2��Һ����������ɫ����������SO42�����˺�ȡ��Һ���μ�H2O2��Һ�����ٲ�����ɫ����������H2SO3
������������C����KSCN��DΪѪ��ɫ��Һ����֪CΪFeCl3��DΪFe(SCN)3�ȣ���֪BΪFe2O3����n(Fe2O3)= ![]() =0.015mol��n(Fe)=0.03mol��m(Fe)=0.03mol��56g/mol=1.68g��Aȼ�����ɵ���ɫ����E��Һˮ�õ�������Һ��������KI��Һ���õ���ɫ��Һ��˵���������E��ˮ��Һ��EӦΪSO2��FΪH2SO3��G���к�H2SO4��HI����֪A����Fe��SԪ�أ���m(S)=2.96g-1.68g=1.28g��n(S)=
=0.015mol��n(Fe)=0.03mol��m(Fe)=0.03mol��56g/mol=1.68g��Aȼ�����ɵ���ɫ����E��Һˮ�õ�������Һ��������KI��Һ���õ���ɫ��Һ��˵���������E��ˮ��Һ��EӦΪSO2��FΪH2SO3��G���к�H2SO4��HI����֪A����Fe��SԪ�أ���m(S)=2.96g-1.68g=1.28g��n(S)= ![]() =0.04mol����֪n(Fe)��n(S)=3��4��ӦΪFe3S4��
=0.04mol����֪n(Fe)��n(S)=3��4��ӦΪFe3S4��
(1)�����Ϸ�����֪��A���Ԫ��ΪFe��S��ΪFe3S4���ʴ�Ϊ��Fe��S��Fe3S4��
(2)�����Ӿ���ǿ�����ԣ�������ͭ��������ӡˢ��·�壬�ʴ�Ϊ��ӡˢ��·�壻
(3)������A����ϡ���ᷴӦ������һ�ֵ���ɫ�������һ������(����µ��ܶ�Ϊ1.518gL-1)������ɫ������ΪS���������Է�������Ϊ1.518��22.4L=34��ΪH2S���壬����ʽΪ![]() ����Ӧ�����ӷ���ʽΪFe3S4+6H+=3Fe2++S+3H2S�����ʴ�Ϊ��
����Ӧ�����ӷ���ʽΪFe3S4+6H+=3Fe2++S+3H2S�����ʴ�Ϊ��![]() ��Fe3S4+6H+=3Fe2++S+3H2S����
��Fe3S4+6H+=3Fe2++S+3H2S����
(4)F��G��Ӧ�Ļ�ѧ����ʽΪH2SO3+I2+H2O=H2SO4+2HI����ҺG�е���Ҫ��(������H2O��H+��K+��I-) ΪSO42-��H2SO3�����ȼ���SO42-���������H2SO3���������Ϊ��ȡ��ҺG���������BaCl2��Һ����������ɫ����������SO42-�����˺�ȡ��Һ���μ�H2O2��Һ�����ٲ�����ɫ����������H2SO3���ʴ�Ϊ��H2SO3+I2+H2O=H2SO4+2HI��ȡ��ҺG���������BaCl2��Һ����������ɫ����������SO42-�����˺�ȡ��Һ���μ�H2O2��Һ�����ٲ�����ɫ����������H2SO3��

����Ŀ��CoxFe3-xO4�ŷ���һ�ֱȽϺõĸ߽������ŷۡ���FeSO4Ϊԭ���Ʊ�CoxFe3-xO4����Ҫ�������£�
![]()
��1�����������FeSO4��Һ�м���NaOH��Һ����40���½�������FeOOH���֡����ɾ��ֵĻ�ѧ����ʽΪ_____________________��
��2������ڽ������Ƶ�����FeSO4��Һ����Ƥ���������У����µ�60������������������ֳ���һ���ߴ���ˡ�ˮϴ�������FeOOH��ĩ���������з�����Ƥ��Ŀ����_______���������������Ϊ_____________________��
��3������۽�FeOOH��200��300���¼�����ˮ�����ɺ�ɫFe2O3��ʵ������ɸò�����Ҫ���������е�___________������ĸ����
a��������b���ձ�����c����������d�������ǡ���e���ƾ���
��4�������ͨ��H2��������300��400��������Fe3O4��ͨ��H2ǰҪ�����¯��ͨ��N2��������Ϊ______________________________________________��
��5��ij�о�С����������ӵ��������������LiCoO2���������������������Ʊ�CoSO4��7H2O���塣�±��г�����ؽ������������������������pH����ʼ������pH����������Ũ��Ϊ1.0 mol��L��1���㣩��
�������� | ��ʼ������pH | ������ȫ��pH |
Fe3�� | 1.1 | 3.2 |
Fe2�� | 5.8 | 8.8 |
Co2�� | 6.9 | 9.4 |
���������ʵ�鲽������ѡ�õ��Լ���H2O2��ϡ���ᡢϡ���ᡢNaOH��Һ����
����N����������ͪ��120���½�ϴ�������ϣ�ʹLiCoO2���������룬�õ�LiCoO2��Ʒ����������
�� _____________________________________________________��
�������ô�ƷCoSO4��Һ�м���NaOH��Һ������pHԼΪ5�����ˡ�
��_______________________________________________________��
�ݽ�Co��OH��2��������ϡ�����У�����Ũ�������½ᾧ���õ�CoSO4��7H2O���塣