ĢāÄæÄŚČŻ
”¾ĢāÄæ”æÄ³ŃŠ¾æŠŌѧĻ°Š”×éĪŖ²ā¶Øijŗ¬Ć¾3%”«5%µÄĀĮĆ¾ŗĻ½š(²»ŗ¬ĘäĖūŌŖĖŲ)ÖŠĆ¾µÄÖŹĮæ·ÖŹż£¬Éč¼ĘĮĖĻĀĮŠČżÖÖ²»Ķ¬ŹµŃé·½°ø½ųŠŠĢ½¾æ£¬Ēėøł¾ŻĖūĆĒµÄÉč¼Ę»Ų“šÓŠ¹ŲĪŹĢā”£
”¾Ģ½¾æŅ»”æ ŹµŃé·½°ø£ŗĀĮĆ¾ŗĻ½š![]() ²ā¶ØŹ£Óą¹ĢĢåÖŹĮ攣
²ā¶ØŹ£Óą¹ĢĢåÖŹĮ攣
ĪŹĢāĢÖĀŪ£ŗ
(1)ŹµŃéÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ________________”£
(2)ČōŹµŃéÖŠ³ĘČ”5.4 gĀĮĆ¾ŗĻ½š·Ūĩѳʷ£¬Ķ¶ČėV mL
2£®0 mol/L NaOHČÜŅŗÖŠ£¬³ä·Ö·“Ó¦”£ŌņNaOHČÜŅŗµÄĢå»żV mL”Ż________mL”£
(3)ŹµŃéÖŠ£¬µ±ĀĮĆ¾ŗĻ½š³ä·Ö·“Ó¦ŗó£¬ŌŚ³ĘĮæŹ£Óą¹ĢĢåÖŹĮæĒ°£¬»¹Šč½ųŠŠµÄŹµŃé²Ł×÷°“Ė³ŠņŅĄ“ĪĪŖ________________”£
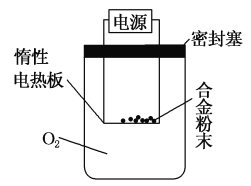
”¾Ģ½¾æ¶ž”æŹµŃé·½°ø£ŗ³ĘĮæx gĀĮĆ¾ŗĻ½š·ŪÄ©£¬·ÅŌŚČēĶ¼ĖłŹ¾ ×°ÖƵĶčŠŌµēČČ°åÉĻ£¬ĶصēŹ¹Ęä³ä·Ö×ĘÉÕ”£
ĪŹĢāĢÖĀŪ£ŗ
(4)Óū¼ĘĖćMgµÄÖŹĮæ·ÖŹż£¬øĆŹµŃéÖŠ»¹Šč²ā¶ØµÄŹż¾ŻŹĒ____________”£
(5)¼ŁÉ菵ŃéÖŠ²ā³öøĆŹż¾ŻĪŖy g£¬ŌņŌĀĮĆ¾ŗĻ½š·ŪÄ©ÖŠĆ¾µÄÖŹĮæ·ÖŹżĪŖ________(ÓĆŗ¬x”¢yµÄ“śŹżŹ½±ķŹ¾)”£
”¾Ģ½¾æČż”æ ŹµŃé·½°ø£ŗĀĮĆ¾ŗĻ½š![]() ²ā¶ØÉś³ÉĘųĢåµÄĢå»ż”£
²ā¶ØÉś³ÉĘųĢåµÄĢå»ż”£
ĪŹĢāĢÖĀŪ£ŗ
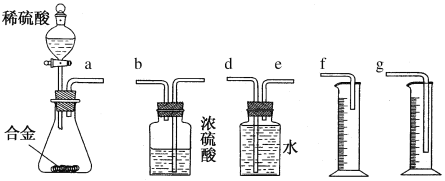
(6)Ķ¬Ń§ĆĒÄāŃ”ÓĆĻĀ±ßµÄŹµŃé×°ÖĆĶź³ÉŹµŃ飬ÄćČĻĪŖ×ī¼ņŅ×µÄ×°ÖƵÄĮ¬½ÓĖ³ŠņŹĒa½Ó______________(Ģī½ÓæŚ×ÖÄø£¬ŅĒĘ÷²»Ņ»¶ØČ«Ń”)”£
”¾“š°ø”æ(1)2Al£«2NaOH£«2H2O=2NaAlO2£«3H2”ü (2)97
(3)¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ¹ĢĢå (4)×ĘÉÕŗó¹ĢĢåµÄÖŹĮæ
(5)![]() (3·Ö) (6)edg
(3·Ö) (6)edg
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗ(1)ĀĮÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ӧɜ³ÉĘ«ĀĮĖįÄĘÓėĒāĘų£¬·“Ó¦·½³ĢŹ½ĪŖ2Al+2NaOH+2H2O=2NaAlO2+3H2”ü£»
(2)ŗ¬Ć¾ĪŖ3%Ź±£¬½šŹōĀĮµÄŗ¬Įæ×īøߣ¬5.4gŗĻ½šÖŠĀĮµÄÖŹĮæĪŖ£¬5.4g”Į(1-3%)=5.4”Į97%g£¬Ōņ£ŗ
2Al+2NaOH+2H2O=2NaAlO2 +3H2ӟ
54g 2mol
5.4g”Į97% V”Į10-3L”Į2.0mol/L
54g£ŗ(5.4g”Į97%)=2mol£ŗ(V”Į10-3L”Į2.0mol/L)£¬
½āµĆ£ŗV=97£¬¹ŹV(NaOHČÜŅŗ)”Ż97mL£»
(3)·“Ó¦ŗó½šŹōĀĮĶźČ«ĻūŗÄ£¬Ć»ÓŠ·“Ó¦µÄĪŖ½šŹōĆ¾£¬³ĘĮ潚ŹōĆ¾Ö®Ē°ŠčŅŖ¾¹ż¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ²Ł×÷£¬Č»ŗóŌŁ³ĘĮæ¹ĢĢåÖŹĮ棬“Ó¶ų¼ĘĖć³öŗĻ½šÖŠĆ¾µÄŗ¬Į棻
(4)Mg”¢Al¾łÓėŃõĘų·“Ó¦£¬Éś³É½šŹōŃõ»ÆĪļ£¬Ōņ»¹Šč²ā¶ØÉś³ÉĪļµÄÖŹĮ棻
(5)ÉčxgĀĮĆ¾ŗĻ½š·ŪÄ©ÖŠŗ¬ÓŠn molĆ¾”¢zmolĀĮ£¬Ōņ24n+27z=x¢Ł£¬
ŌŁøł¾Ż·“Ó¦¹ŲĻµŹ½£ŗMg”«MgO”¢Al”«Al2O3£¬×īŗóµĆµ½ĮĖygŃõ»ÆĪļ£¬¾Ż“ĖĮŠŹ½ĪŖ£ŗ40n+51z=y¢Ś£¬øł¾Ż¢Ł¢Ś½āµĆ£ŗz=(3y5x)”Ā18mol£¬ĀĮµÄÖŹĮæĪŖ£ŗ27g/mol”Į(3y5x)”Ā18mol=![]() g£¬ŗĻ½šÖŠĆ¾µÄÖŹĮæ·ÖŹżĪŖ
g£¬ŗĻ½šÖŠĆ¾µÄÖŹĮæ·ÖŹżĪŖ![]() £»
£»
(6)×°ÖƵÄ×é×°Ė³Šņ£ŗŗĻ½šÓėĖį·“Ó¦£¬ÓĆÅÅĖ®ĮæĘų·Ø²ā¶ØĒāĘųµÄĢå»ż£¬ĘäÖŠŹ¢Ė®µÄŹŌ¼ĮĘæµ¼¹ÜŅ»¶ØŅŖ¶Ģ½ų³¤³ö£¬ĄūÓĆŌö“óŃ¹ĒæŌĄķ½«Ė®Åųö£¬ĮæĶ²ÖŠĖ®µÄĢå»ż¾ĶŹĒÉś³ÉĒāĘųµÄĢå»ż£¬ĮæĶ²ÄŚµ¼¹ÜÓ¦ÉģČėĮæĶ²µ×²æ£¬¹ŹĮ¬½ÓĖ³ŠņĪŖa½Óe”¢d½Óg”£

 æŚĖćĢāæر±¾©ø¾Å®¶łĶƳö°ęÉēĻµĮŠ“š°ø
æŚĖćĢāæر±¾©ø¾Å®¶łĶƳö°ęÉēĻµĮŠ“š°ø”¾ĢāÄæ”æŅ»¶ØĪĀ¶ČĻĀ£¬ŌŚČżøöĢå»ż¾łĪŖ1.0 LµÄŗćČŻĆܱÕČŻĘ÷ÖŠ£¬³äČėŅ»¶ØĮæµÄH2ŗĶSO2·¢ÉśĻĀĮŠ·“Ó¦£ŗ3 H2£Øg£© + SO2£Øg£©![]() 2 H2O£Øg£© + H2S£Øg£©£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø £©
2 H2O£Øg£© + H2S£Øg£©£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø £©
ČŻĘ÷±ąŗÅ | ĪĀ¶Č/”ę | ĘšŹ¼ĪļÖŹµÄĮæ/mol | Ę½ŗāĪļÖŹµÄĮæ/mol | ||
H2 | SO2 | H2 | SO2 | ||
ČŻĘ÷¢ń | 300 | 0.3 | 0.1 | 0.02 | |
ČŻĘ÷¢ņ | 300 | 0.6 | 0.2 | ||
ČŻĘ÷¢ó | 240 | 0.3 | 0.1 | 0.01 | |
A£®øĆ·“Ó¦Õż·“Ó¦ĪŖĪüČČ·“Ó¦
B£®ČŻĘ÷¢ó“ļµ½Ę½ŗāµÄŹ±¼ä±ČČŻĘ÷I¶Ģ
C£®240”ꏱ£¬øĆ·“Ó¦µÄĘ½ŗā³£ŹżĪŖ1.08”Į104
D£®ČŻĘ÷¢ņ“ļµ½Ę½ŗāŹ±SO2µÄ×Ŗ»ÆĀŹ±ČČŻĘ÷IŠ”