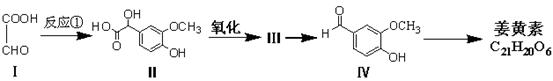
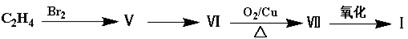��Ŀ����
���ɱ��������ȩ�����ʺϳ�F���ϳ�·�����£�
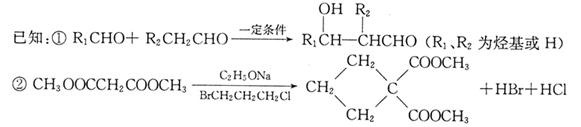
��ش��������⣺
��1��A�Ĺ����ŵ�����Ϊ ������ϳɷ��������Ʋ�C�Ľṹ��ʽ ��
��2��д����Ӧ�ܵĻ�ѧ����ʽ ����Ӧ����Ϊ ��
��3��д����������������D��ͬ���칹��Ľṹ��ʽ ��
����D������ȫ��ͬ�Ĺ�����
��ÿ��̼�����ֻ����һ��������
�ۺ˴Ź���������5�����շ�
��4����E���ڷ�̪ϡ��Һ�����Թ۲쵽�������� ����֪F���ʺɱ����ֵΪ384����E�������ºϳ�F�Ļ�ѧ��Ӧ����ʽΪ ��


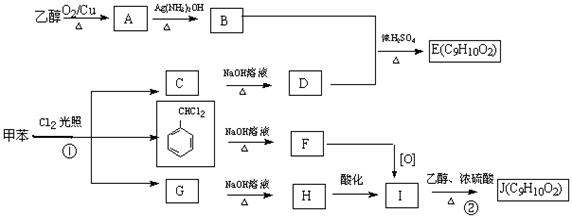
��ش��������⣺
��1��A�Ĺ����ŵ�����Ϊ ������ϳɷ��������Ʋ�C�Ľṹ��ʽ ��
��2��д����Ӧ�ܵĻ�ѧ����ʽ ����Ӧ����Ϊ ��
��3��д����������������D��ͬ���칹��Ľṹ��ʽ ��
����D������ȫ��ͬ�Ĺ�����
��ÿ��̼�����ֻ����һ��������
�ۺ˴Ź���������5�����շ�
��4����E���ڷ�̪ϡ��Һ�����Թ۲쵽�������� ����֪F���ʺɱ����ֵΪ384����E�������ºϳ�F�Ļ�ѧ��Ӧ����ʽΪ ��
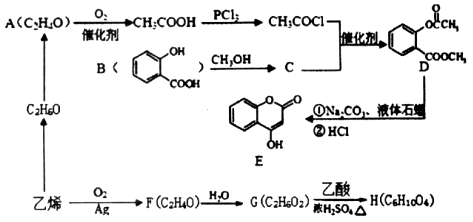
��1���ǻ�
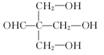
��2��
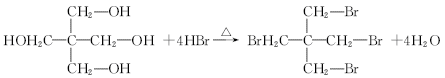 ȡ����Ӧ
ȡ����Ӧ��3��
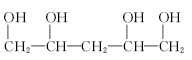
��4����ɫ��̪��ɺ�ɫ
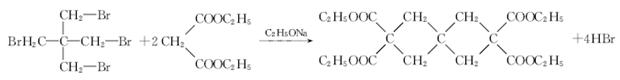
�����������1��ȩ�����Ϊ�������еĹ�����Ϊ�ǻ�����ȩ��C�ķ�Ӧʵ��Ϊȩ��ȩ�ļӳɣ��������Ʒ�������̼�Ǽ�û�䣬CΪ̼ԭ����Ϊ5��ȩ����2����Ӧ��Ϊ����±������ȡ����Ӧ����3��D�ķ���ʽΪC5H12O4������4�����ǻ����ṹ�߶ȶԳƣ�����˴�������5�����շ壬����д����ؽṹ��ʽ��������ˮ�ᷢ������ˮ�⣬���ɴ����������ƣ���Һ�Լ��ԣ���ɫ��̪��Ϊ��ɫ�����ݷ�Ӧ��������ﲻ��д����ػ�ѧ����ʽ��

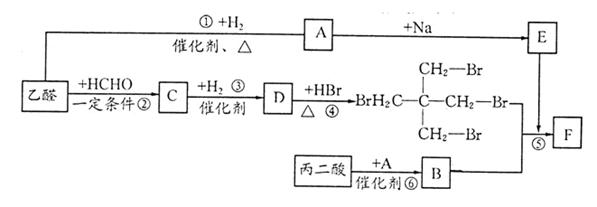
��ϰ��ϵ�д�
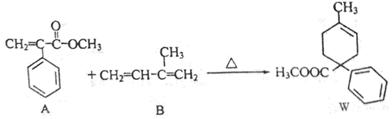
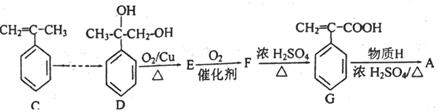
�����Ŀ
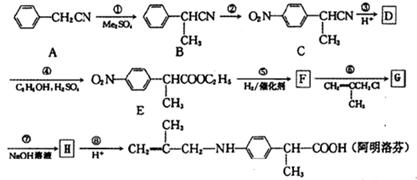
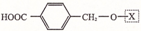 ��Ϊ���о�X�Ľṹ����������A��һ��������ˮ��ֻ�õ�B������ʽΪC8H8O3����C������ʽΪC7H6O3����C��FeCl3ˮ��Һ����ɫ����NaHCO3��Һ��Ӧ��CO2������
��Ϊ���о�X�Ľṹ����������A��һ��������ˮ��ֻ�õ�B������ʽΪC8H8O3����C������ʽΪC7H6O3����C��FeCl3ˮ��Һ����ɫ����NaHCO3��Һ��Ӧ��CO2������
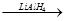 RCH2OH
RCH2OH R��COOH
R��COOH ��Ϊ��Ҫԭ���Ʊ�
��Ϊ��Ҫԭ���Ʊ�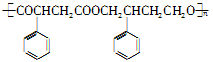 �ĺ�
�ĺ� H2C=CH2
H2C=CH2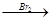 H2CBr��CH2 Br
H2CBr��CH2 Br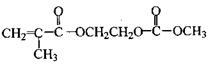

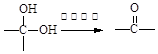
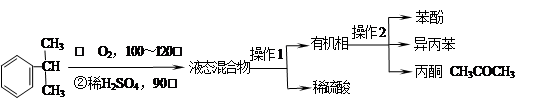
 + 28KMnO4 + 42H2SO4
+ 28KMnO4 + 42H2SO4 28MnSO4 + 14K2SO4 + 30CO2��+ 57H2O
28MnSO4 + 14K2SO4 + 30CO2��+ 57H2O