��Ŀ����
13��ʵ�����Ʊ�����ͪ�ķ�Ӧԭ��Ϊ��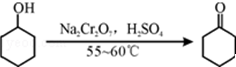 �䷴Ӧ��װ��ʾ��ͼ��ͼ1���г�װ�á�����װ����ȥ����������������ͪ������ʳ��ˮ��ˮ�IJ����������ʼ��±���
�䷴Ӧ��װ��ʾ��ͼ��ͼ1���г�װ�á�����װ����ȥ����������������ͪ������ʳ��ˮ��ˮ�IJ����������ʼ��±���| ���� | �е㣨�棩 | �ܶȣ�g•cm-3��20�棩 | �ܽ��� |
| ������ | 161.1��97.8�� | 0.9624 | ������ˮ |
| ����ͪ | 155.6��95�� | 0.9478 | ����ˮ |
| ����ʳ��ˮ | 108.0 | 1.3301 | |
| ˮ | 100.0 | 0.9982 |
��1��ʵ����ͨ��װ��B������Na2Cr2O7��Һ�ӵ�ʢ�л�������A�У���55��60����з�Ӧ����Ӧ��ɺ�������ˮ�������ռ�95��100�����֣��õ���Ҫ������ͪ��Ʒ��ˮ�Ļ����
������B�������Ƿ�Һ©����
���������ʱ��һ��ʱ�����δͨ����ˮ��Ӧ��ȡ����ȷ������ֹͣ���ȣ���ȴ��ͨ����ˮ��
�������ܷ��뻷��ͪ��ˮ��ԭ���ǻ���ͪ��ˮ�γɾ��й̶���ɵĺ����һ��������
��2����Ư�۾��ͱ������������Na2Cr2O7��ҺҲ�������������ƻ���ͪ����Ư�۾��ͱ���������ͻ�����ŵ��DZ���ʹ���ж���Na2Cr2O7��
��3������ͪ���ᴿ��Ҫ��������һϵ�еIJ�����
a�������ռ�151��156����֣��õ���Ʒ b������
c�����ռ����Ĵ�Ʒ�м�NaCl���������ͣ����ã���Һ
d��������ˮMgSO4���壬��ȥ�л���������ˮ
��������������ȷ˳����c d b a������ţ�
������������c�У�����NaCl���������������ˮ����ܶȣ������ڷֲ㣬��С�������ʧ��
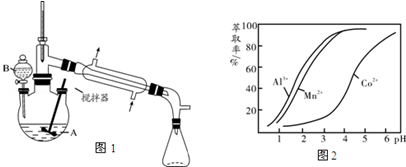
��4������ͪ��һ�ֳ��õ���ȡ�����Ի���ͪΪ�ɷ�֮һ����ȡҺ�Խ������ӵ���ȡ����pH�Ĺ�ϵ��ͼ2��ʾ������ij�ܿ�ʯ���������Һ������Һ���е���������Ҫ��H+��Co2+��Mn2+��Al3+����ȥAl3+����������ȡ�������MnCl2�Եõ���Ϊ������CoCl2��Һ��pH��ΧӦ������b��
a��2.0��2.5 b��3.03.5 c��4.0��4.5��
���� ��1���ٸ��������Ĺ�����ص����ش�
���������ʱ��һ��ʱ�����δͨ����ˮ��Ϊ��ֹ������ը�ѣ�Ӧ�õȵ�װ����ȴ����ͨ����ˮ��
�۸��ݻ���ͪ��ˮ�γɵľ��й̶���ɵĻ����ķе��ˮ�ķе�������ش�
��2���ظ�������һ���ж����ʣ�������Һ����ǿ�����ԣ��ݴ˻ش�
��3������ͪ���ᴿʱӦ���ȼ���NaCl���壬ʹˮ��Һ���ܶ�����ˮ���л���������뿪����Ȼ�����л����м�����ˮMgSO4����ȥ�л�����������ˮ��Ȼ����ˣ���ȥ����þ���壬�ٽ������ɣ�
��4���ɱ������ݿ�֪��������ҺPH��3.0��3.5֮�䣬��ʹMn2+��ȫ����������ֹCo2+ת��ΪCo��OH��2������
��� �⣺��1��������B�������Ƿ�Һ©�����ʴ�Ϊ����Һ©����
���������ʱ��һ��ʱ�����δͨ����ˮ������������ͨ����ˮ����ֹ���佫������ը�ѣ�Ӧ�õȵ�װ����ȴ����ͨ����ˮ��
�ʴ�Ϊ��ֹͣ���ȣ���ȴ��ͨ����ˮ��
�ۻ���ͪ��ˮ���γɾ��й̶���ɵĻ������й̶��ķе㣬����ʱ�ܱ�һ�������������������Է��뻷��ͪ��ˮ�Ļ�������ͪ��ˮ�ܹ��������У���ȡ�����Dz���ȡ�ģ�������þ���
�ʴ�Ϊ������ͪ��ˮ�γɾ��й̶���ɵĺ����һ��������
��2��Ư�۾��ͱ����ᷴӦ���ɵĴ�������н�ǿ�������ԣ�ʹ��Ư�۾��ͱ����������棬��������ʹ���ж���Na2Cr2O7��
�ʴ�Ϊ������ʹ���ж���Na2Cr2O7��
��3������ϵ��ȡ����������ԭ����������ˮ����ܶȣ������ڷֲ㣬����ͪ���ᴿʱӦ���ȼ���NaCl���壬ʹˮ��Һ���ܶ�����ˮ���л���������뿪����Ȼ�����л����м�����ˮMgSO4����ȥ�л�����������ˮ��Ȼ����ˣ���ȥ����þ���壬�ٽ������ɣ�
�ʴ�Ϊ��c d b a��
�ڼ���NaCl���壬ʹˮ��Һ���ܶ�����ˮ���л���������뿪����
�ʴ�Ϊ������ˮ����ܶȣ������ڷֲ㣬��С�������ʧ��
��4������ȡ���Խ������ӵ���ȡ����pH�Ĺ�ϵ��֪��������ҺPH��3.0��3.5֮�䣬��ʹMn2+��ȫ����������ֹCo2+ת��ΪCo��OH��2������
�ʴ�Ϊ��b��
���� ����ͨ����ȡ�Ʊ�����ͪ�Ĺ������̣������������Ʊ���������ƣ���Ŀ�Ѷ��еȣ����������ͼ����ȷʵ���������Ƽ�������ʵ������ǽ����Ĺؼ��������ֿ�����ѧ���ķ������������������Ӧ����ѧ֪ʶ��������

| A�� | Fe2O3 | B�� | NaCl | C�� | Cu2S | D�� | Al2O3 |
| A�� | pH=2��HA��Һ��pH=12��MOH��ǿ���Һ����������Һһ���Լ��� | |
| B�� | ���ʵ���Ũ����ȵ�CH3COONa��NaOH��Na2CO3������Һ��pH��NaOH����pH��CH3COONa����pH��Na2CO3�� | |
| C�� | NaHCO3��Һ�У�c��OH-��-c��H+��=c��H2CO3��-c��CO32-�� | |
| D�� | 10��ʱpH=12��NaOH��Һ��40��ʱpH=12��NaOH��Һ�У�c��OH-����� |
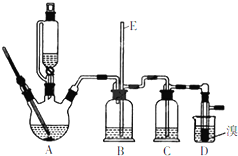 ʵ����������������������Ҵ��Ʊ�1��2-���������װ����ͼ��ʾ�����ȼ��г�װ��ʡ�ԣ���
ʵ����������������������Ҵ��Ʊ�1��2-���������װ����ͼ��ʾ�����ȼ��г�װ��ʡ�ԣ����Ʊ�1��2--��������ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������140����ˮ�������ѣ��й������б����£�
| �Ҵ� | 1��2-�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g��cm-3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 | 132 | 34.6 |
| �۵�/�� | -130 | 9 | -116 |
��1��װ��A��D�з�����Ҫ��Ӧ�Ļ�ѧ����ʽΪ��CH3CH2OH $��_{170��}^{Ũ����}$CH2=CH2��+H2O��CH2=CH2+Br-Br��CH2Br-CH2Br��
��2��װ��B�г�������E�����ã��ж�װ���Ƿ������
��3���ڴ��Ʊ�ʵ���У�Ҫ������Ѹ�ٵذѷ�Ӧ�¶���ߵ�170�����ң�������ҪĿ����d��������ȷѡ��ǰ����ĸ��
a��������Ӧ b���ӿ췴Ӧ�ٶ� c����ֹ�Ҵ��ӷ� d�����ٸ�������������
��4����װ��C��Ӧ����c����Ŀ������ȫ���շ�Ӧ�п������ɵ��������壺������ȷѡ��ǰ����ĸ��
a��ˮ b��Ũ���� c������������Һ d������KMn04��Һ
��5����Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ���ǣ����ӷ�����ˮ�ɼ��ٻӷ������ֲ��ܹ��ȣ���ȴ�����ñ�ˮ������ԭ���ǣ�����ñ�ˮ��ȴ��ʹ��Ʒ���̶��������ܣ���1��2-��������ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã�����Ӧ���²㣨��ϡ������¡�����
| A | B | c | D | |
| �� Ʒ |  |  |  |  |
| ��Ҫ�ɷ� | CO2 | Fe2O3 | NaHCO3 | C12H22011 |
| ��; | ������� | ����ɫͿ�� | ������ | ����ζ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ��ˮ����ͭ | B�� | �������� | C�� | ʳ�� | D�� | ��ʯ�� |
| A�� | ��ʪ���pH��ֽ��ϡ����Һ��pH���ⶨֵƫС | |
| B�� | ������ƿ������Һ������ʱ���ӿ̶��ߣ�������ҺŨ��ƫС | |
| C�� | ����ƿ�к�����������ˮ���������Һ��Ӱ�� | |
| D�� | �ⶨ�кͷ�Ӧ���к���ʱ������Ч��Խ�ã��ⶨ���ԽС |
�ٱ����������������屽�������Ȼ�̼��
| A�� | �٢ۢ� | B�� | �ڢۢ� | C�� | �٢� | D�� | ȫ�� |