��Ŀ����
����Ŀ��������ͬѧ�ֱ�Ժ�+4����Ԫ�ص��������ʽ�����̽����
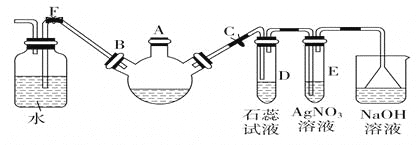
��1��������ͼװ�ý���ʵ��(�������Ѽ��������Ⱥͼг�װ������ȥ)��ʵ�����һ��ʱ���C��D�ж��������Ե���ɫ�������������ΪBaSO4��
�� A�з�Ӧ�Ļ�ѧ����ʽ��______________��
��Ϊ̽��SO2��D���������ķ�Ӧ����һ��ʵ�鷢�֣�������ɫ�����Ĺ����У�D��Һ��NO3-Ũ�ȼ������䡣�ݴ˵ó����ۣ�D�г�����ɫ��������Ҫԭ����__________________��
��2����������ʵ��Ժ�+4����Ԫ�ص��������ʼ�������̽����
���� | ʵ������ | ʵ������ |
1 | ȡ0.3g����Na2SO3���壬�����м���10mL 2 mol/L������������4��BaCl2��Һ | ������ɫ����������BaCl2��Һ��ʼ������4 min����Һ����� |
2 | ȡ0.3g����Na2SO3�����������м���10mL 2 mol/L HNO3���ٵ���4��BaCl2��Һ | ������ɫ����������BaCl2��Һ������ʼ��������2 h����Һ����� |
3 | ȡ0.3g����Na2SO3���壬�����м���10mL ŨHNO3,������4��BaCl2��Һ | ��������ɫ����������BaCl2��Һ������Һ��������������ɫ���� |
�������ӷ���ʽ����ʵ��1�в��������ԭ��________________��
�� ��ʵ��1��2��3�Աȣ����Եõ�����:________________��
����ͨ���������Ϸ���.Na+��ʵ��1��2�г��ֻ��ǵ�ʱ����Ӱ�죬���ǽ�һ��̽��Cl-�� NO3-������Ӱ�죺
��� | ʵ������ | ʵ������ |
4 | ȡ____�������������м���10mL2 mol /LHNO3���ٵ���4��BaCl2��Һ | ������ɫ������������BaCl2��Һ����ʼ��������20 min����Һ����� |
i.ʵ��2��4�Աȣ��һ������:C1-�Ĵ��ڿ��Լӿ���Һ��+4����Ԫ����������
ii.ʵ��I��4�Աȣ��һ������: ______________��
��ͨ������ʵ�飬��ͬѧ��Ϊ��ȷ��ij��Һ�к���SO42-��ʵ�鷽����ȡ����Һ���������ȵμ�____________ (����ĸ���)��
a��2 mol/L���ᣬ�ٵμ�BaCl2��Һ��һ��ʱ�����ְ�ɫ����
B��2 mol/L�������ٵμ�BaCl2��Һ���������ְ�ɫ����
C��2 mol/L���ᣬ�ٵμ�BaCl2��Һ��һ��ʱ��������ɫ����
D��2 mol/L���ᣬ�ٵμ�BaCl2��Һ������������ɫ����
���𰸡�
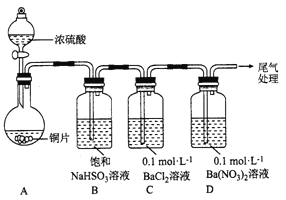
��1����Cu+2H2SO4(Ũ��![]() CuSO4+SO2��+2H2O�������Ǽ��ȣ�
CuSO4+SO2��+2H2O�������Ǽ��ȣ�
�����������£���+4����Ԫ������(SO2��H2SO3)��O2��������SO42-
��2����2H++SO32-=SO2+H2O��
SO2+O2+2Ba2++2H2O=2BaSO4��+4H+��
����2H2SO3+O2+2Ba2+=2BaSO4��+4H+��
����+4����Ԫ�����ʿɱ�O2��ŨHNO3����
��0.3g����Na2SO3��1.17gNaCl
ii.NO3-�Ĵ��ڿ��Լ�����Һ��+4����Ԫ�ص�������bd
��������
�����������1����ͭ��Ũ������ȷ�����Ӧ��������ͭ�����������ˮ����Ӧ�Ļ�ѧ����ʽΪ��Cu+2H2SO4(Ũ)![]() CuSO4+SO2��+2H2O��
CuSO4+SO2��+2H2O��
��Ϊ̽��SO2��D���������ķ�Ӧ����һ��ʵ�鷢�֣����ְ�ɫ�����Ĺ����У�D��Һ��NO3-Ũ�ȼ������䣮�ݴ˵ó����ۣ�D�г��ְ�ɫ��������Ҫԭ����������Һ�ж�������ᱻ���������������ᣬ��ϱ����ӣ�Ҳ���������ᱵ������
��2����ȡ0.3g ����Na2SO3���壬�����м���10mL 2molL-1 ���ᣬ�ٵ���4��BaCl2��Һ��������ɫ����Ϊ�����������壬����BaCl2��Һ��ʼ������4min����Һ����ǣ�˵�����������������������������ᣬ��ϱ������������ᱵ��ɫ��������Ӧ�����ӷ���ʽΪ��2H++SO32-��SO2+H2O��2SO2+O2+2Ba2++2H2O��2BaSO4��+4H+��2H2SO3+O2+2Ba2+��2BaSO4��+4H+��
����ʵ��1˵������������Ҳ��������+4����Ԫ�صĻ����ʵ��2˵��������Һ����������Ӷ�������Ӧ�������ã����ֳ���ʱ�䳤��ʵ��3��Ũ����������+4����Ԫ�ػ�����������������ӣ����ֳ����죬�Աȿ�֪������Ũ���ᶼ����������������
��̽��Cl-��NO3-�����Ӱ�죬i��ʵ��2��4�Աȣ��һ�����ۣ�Cl-�Ĵ��ڿ��Լӿ���Һ��+4����Ԫ�ص�������ʵ��4����Ҫ�ṩ��ʵ��1����ͬ��������ʵ��̽��������Ҫ0.01L��2mol/L��0.02mol���Ȼ��Ƶ�������0.02mol��58.5g/mol��1.17g���Ա�ʵ��1�жϳ��ֳ�����ʱ�������ȡ0.3g ����Na2SO3��1.17gNaCl���壬�����м���10mL 2molL-1 HNO3���ٵ���4��BaCl2��Һ���۲���ֳ�����ʱ�䣻
ii��ʵ��1��4�Աȣ���ͬ������������ᣬ��������ͬ�����ֳ�����ʱ����������Һ�п죬�һ����������������Ӽ���+4����Ļ������������ʵ��1��4�Աȣ��һ�������ǣ�NO3-�Ĵ��ڿ��Լ�����Һ��+4����Ԫ�ص�������
���Ա�����ʵ��ȷ��ij��Һ�к���SO42-��ʵ�鷽���ǣ�ʵ��1��֪��ȡ0.3g ����Na2SO3���壬�����м���10mL 2molL-1 ���ᣬ�ٵ���4��BaCl2��Һ��������ɫ���ݣ�����BaCl2��Һ��ʼ������4min����Һ����ǣ�������������ӣ�����������Ȼ�����Һ��Ѹ�����ɰ�ɫ������ʵ��2��֪��ȡ0.3g ����Na2SO3���壬�����м���10mL 2molL-1 HNO3���ٵ���4��BaCl2��Һ��������ɫ���ݣ�����BaCl2��Һ��ʼ������2h����Һ����ǣ�����������Ȼ�����Һ��+4����Ԫ�ػ��ϼ۱����������ʼ�����������������ӻ�Ѹ�����ɳ�����

����Ŀ������ѧ����ѡ��3�����ʽṹ�����ʡ�T��W��X��Y��Z��Ԫ�����ڱ�ǰ�������еij���Ԫ�أ�ԭ�������������������Ϣ���±���
Ԫ�� | �����Ϣ |
T | TԪ�ؿ��γ���Ȼ��Ӳ�����ĵ��� |
W | W��Tͬ���ڣ�������һ��δ�ɶԵ��� |
X | Xԭ�ӵĵ�һ���������ĵ����ֱܷ�I1="578" kJ/mol�� I2=" l817" kJ/mol��I3="2745" kJ/mol��I4=11575kJ/mol |
Y | ���³�ѹ�£�Y�����ǹ��壬�����������γ��������Ҫ���� |
Z | Z��һ��ͬλ�ص�������Ϊ63��������Ϊ34 |
��1�� TY2��һ�ֳ��õ��ܼ�����__________(�������Է����������Ǽ��Է��������������д���________��������
��2��W������⻯������Һ����������__________��.����419 kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ __________��
��3����̬Yԭ���У�����ռ�ݵ�����ܲ����Ϊ__________�����ܲ���е�ԭ�ӹ����Ϊ_____________��������Ϊ_________��Y������WԪ�صĵ�һ�������ɴ�С��˳��Ϊ_________(��Ԫ�ط�������)��
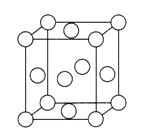
��4����֪Z�ľ����ṹ��ͼ��ʾ����֪Z���ܶ�Ϊ9.00 g/cm3�����߳�Ϊ___________cm��ZYO4�������Һ������ZYO42-�Ŀռ乹����__________������Yԭ�ӵ��ӻ����������___________��Ԫ��Z������������е������Լ�������Ӧ�����ɳ����Z +HCl+O2=ZC1+HO2��HO2(������)������һ���������Ҳ��һ�����ɻ������м��ߵĻ��ԡ�����˵�����ʾ��ȷ����
A��O2��������
B��HO2����������
C��HO2�ڼ������ȶ�����
D��1 mol Z�μӷ�Ӧ��1 mol���ӷ���ת��