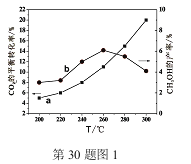
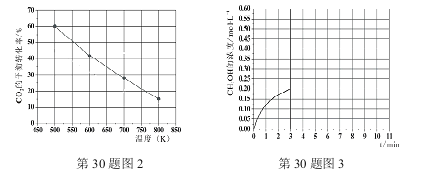
��Ŀ����
����Ŀ������NaHCO3��Na2CO3��xH2O�Ļ���Ϊ�˲ⶨxֵ��ijͬѧ������ͼ��ʾ��װ�ý���ʵ��(CaCl2����ʯ�Ҿ�����)��

(1)Aװ�õ�������____________________________��
(2)Bװ�õ�������____________________________��
(3)Cװ�õ�������___________________________��
(4)����װ��A���Թ���װ��NaHCO3��Na2CO3��xH2O�Ļ����3.7 g���þƾ��Ƽ��ȵ���Ӧ��ȫ����ʱB������1.89 g��C������0.22 g����x��ֵΪ__________________��
(5)��װ�û����Ǻ����ƣ�����ʹ�ⶨ���ƫС��Ӧ��θĽ�________________��Ϊʲô��_________________________��
���𰸡�(10��)(1)������ʹNaHCO3�ֽ���ʹNa2CO3��H2Oʧˮ(1��)
(2)���շ�Ӧ�����ɵ�ˮ(1��)(3)���շ�Ӧ�����ɵ�CO2(1��)(4)10(3��)
(5)��Cװ�ú��ټ�һ��װ�м�ʯ�ҵ�U�ι�(2��)��Ϊ�˷�ֹ�����е�CO2��H2O��Cװ�õ��еļ�ʯ������(1��)
��������
���������(1)�ڼ��ȵ�������̼�������ֽ�����̼���ơ�ˮ��CO2����̼���ƾ���Ҳ��ʧȥ�ᾧˮ������Aװ�õ������Ǽ��ȣ�ʹNaHCO3�ֽ⣬ʹNa2CO3H2Oʧˮ��
(2)��ˮ�Ȼ���������ˮ������ͨ��������Ӧǰ����������Եó�ˮ���������������Bװ�õ����������շ�Ӧ�����ɵ�ˮ��
(3)���ڷ�Ӧ�л���CO2���ɣ�����ʯ�ҿ�������CO2�����ͨ��������Ӧǰ������������Եó�CO2������������Cװ�õ����������շ�Ӧ�����ɵ�CO2��
(4)�þƾ��Ƽ��ȵ���Ӧ��ȫ����ʱB������1.89g��C������0.22 g����˵����Ӧ�����ɵ�ˮ��������=1.89g��CO2����=0.22g�����ʵ����ֱ���![]() =0.105mol��
=0.105mol��![]() =0.005mol�����ݷ�Ӧ�ķ���ʽ2NaHCO3
=0.005mol�����ݷ�Ӧ�ķ���ʽ2NaHCO3![]() Na2CO3+H2O+CO2����֪���÷�Ӧ�����ɵ�CO2����0.005mol����̼�����Ƶ����ʵ�����0.005mol��2=0.01mol����Ӧ�����ɵ�ˮ��0.005mol����̼�����ڷ�Ӧ��ʧȥ��ˮ�����ʵ���=0.105mol-0.005mol=0.100mol��̼�����Ƶ�����=0.01mol��84g/mol=0.84g������Ʒ��̼���ƾ��������=3.7g-0.84g=2.86g����
Na2CO3+H2O+CO2����֪���÷�Ӧ�����ɵ�CO2����0.005mol����̼�����Ƶ����ʵ�����0.005mol��2=0.01mol����Ӧ�����ɵ�ˮ��0.005mol����̼�����ڷ�Ӧ��ʧȥ��ˮ�����ʵ���=0.105mol-0.005mol=0.100mol��̼�����Ƶ�����=0.01mol��84g/mol=0.84g������Ʒ��̼���ƾ��������=3.7g-0.84g=2.86g����![]() ��x=0.1mol�����x=10��
��x=0.1mol�����x=10��
(5)���ڿ�����Ҳ����CO2��ˮ������Ҳ�ܱ�Cװ�õ��м�ʯ�����գ��Ӷ�����C������ƫ�ߣ���˲���ֵƫ�ͣ����ԸĽ��Ĵ�ʩΪ��Cװ�ú��ټ�һ��װ�м�ʯ�ҵ�U�ιܣ�Ϊ�˷�ֹ�����е�CO2��H2O��Cװ�õ��еļ�ʯ��������

����Ŀ��������ͬѧ�ֱ�Ժ�+4����Ԫ�ص��������ʽ�����̽����
��1��������ͼװ�ý���ʵ��(�������Ѽ��������Ⱥͼг�װ������ȥ)��ʵ�����һ��ʱ���C��D�ж��������Ե���ɫ�������������ΪBaSO4��
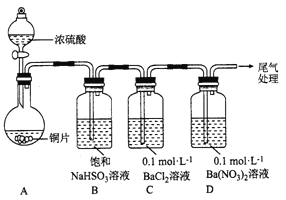
�� A�з�Ӧ�Ļ�ѧ����ʽ��______________��
��Ϊ̽��SO2��D���������ķ�Ӧ����һ��ʵ�鷢�֣�������ɫ�����Ĺ����У�D��Һ��NO3-Ũ�ȼ������䡣�ݴ˵ó����ۣ�D�г�����ɫ��������Ҫԭ����__________________��
��2����������ʵ��Ժ�+4����Ԫ�ص��������ʼ�������̽����
���� | ʵ������ | ʵ������ |
1 | ȡ0.3g����Na2SO3���壬�����м���10mL 2 mol/L������������4��BaCl2��Һ | ������ɫ����������BaCl2��Һ��ʼ������4 min����Һ����� |
2 | ȡ0.3g����Na2SO3�����������м���10mL 2 mol/L HNO3���ٵ���4��BaCl2��Һ | ������ɫ����������BaCl2��Һ������ʼ��������2 h����Һ����� |
3 | ȡ0.3g����Na2SO3���壬�����м���10mL ŨHNO3,������4��BaCl2��Һ | ��������ɫ����������BaCl2��Һ������Һ��������������ɫ���� |
�������ӷ���ʽ����ʵ��1�в��������ԭ��________________��
�� ��ʵ��1��2��3�Աȣ����Եõ�����:________________��
����ͨ���������Ϸ���.Na+��ʵ��1��2�г��ֻ��ǵ�ʱ����Ӱ�죬���ǽ�һ��̽��Cl-�� NO3-������Ӱ�죺
��� | ʵ������ | ʵ������ |
4 | ȡ____�������������м���10mL2 mol /LHNO3���ٵ���4��BaCl2��Һ | ������ɫ������������BaCl2��Һ����ʼ��������20 min����Һ����� |
i.ʵ��2��4�Աȣ��һ������:C1-�Ĵ��ڿ��Լӿ���Һ��+4����Ԫ����������
ii.ʵ��I��4�Աȣ��һ������: ______________��
��ͨ������ʵ�飬��ͬѧ��Ϊ��ȷ��ij��Һ�к���SO42-��ʵ�鷽����ȡ����Һ���������ȵμ�____________ (����ĸ���)��
a��2 mol/L���ᣬ�ٵμ�BaCl2��Һ��һ��ʱ�����ְ�ɫ����
B��2 mol/L�������ٵμ�BaCl2��Һ���������ְ�ɫ����
C��2 mol/L���ᣬ�ٵμ�BaCl2��Һ��һ��ʱ��������ɫ����
D��2 mol/L���ᣬ�ٵμ�BaCl2��Һ������������ɫ����