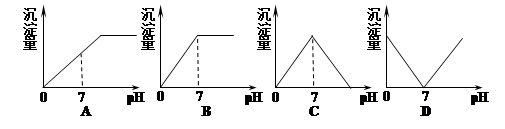��Ŀ����
���л�ѧ���̼��������ȷ���� �� ��
| A����NaHSO4��Һ�е���Ba(OH)2��Һ������H+��SO42-��Ba2+��OH-��BaSO4����H2O |
| B����ˮ�����c(H+)Ϊ10-3 mol��L-1����Һ�У�Na+��NO3-��SO32-��Cl-һ���ܴ������� |
| C������1molKAl(SO4)2����Һ�м���Ba(OH)2��Һ�������������ʱ���������ܵ����ʵ���Ϊ2mol |
| D��������Ũ�����ữ��KMnO4��Һ��H2O2��ϣ���֤��H2O2���л�ԭ��2MnO4-��6H+��5H2O2��2Mn2+��5O2����8H2O |
C
�������������
���⿼��������������������ӹ��漰�����йص����ӷ���ʽ��д��A.Ҫʹ����Һ�����ԣ���ô
H+��OH-��������1:1��1molNaHSO4�����1molH+��1molBa(OH)2�����2molOH-������n(NaHSO4):n[Ba(OH)2]=2:1���������ӷ���ʽӦΪ��2H+��SO42-��Ba2+��2OH-��BaSO4����2H2O��B����ˮ�����c(H+)Ϊ10-3 mol��L-1��˵������Һ�Ǵٽ���ˮ�ĵ��롣ˮ��c(H+)=10-7mol��L-1�����Ը���Һ�бغ������������ӣ�Fe3+��Cu2+ ��Al3+�ȣ�����������ӣ�CO32-��CH3COO-�ȣ��������Һ�д���Fe3+��Cu2+�Ļ�����ô��Ȼ��SO32-���ܴ������档D��KMnO4��Һ������Ũ�����ữ������������KMnO4��Һ����Ũ���ᷢ����Ӧ��Ҳ������ŨHNO3�ữ����ŨHNO3��������ǿ�����ԣ���C��Al3++2SO42-+ 2Ba2+��4OH-= 2BaSO4��+AlO2-+2H2O�����������ʵ���Ϊ2mol������Ϊ466g��Al3++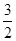 SO42-+
SO42-+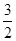 Ba2+��3OH-=
Ba2+��3OH-=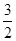 BaSO4��+Al(OH)3������������£����������ʵ���Ϊ2.5mol��������Ϊ427.5g������C��ȷ��
BaSO4��+Al(OH)3������������£����������ʵ���Ϊ2.5mol��������Ϊ427.5g������C��ȷ��
���㣺�����������������йأ������ӷ���ʽ����д

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д��������ӻ��������Һ���ܴ������棬ͨ��CO2�����ܴ��������һ����
| A��K+��Na+��Br����SiO32�� | B��H+��Fe2+��SO42����Cl2 |
| C��K+��Ba2+��Cl����NO3�� | D��K+��Ag+��NH3��H2O��NO3�� |
�ڲ�ͬ�¶��£�ˮ��Һ��c(H+)��c(OH-)����ͼ��ʾ��ϵ�����������������ӹ���˵������ȷ����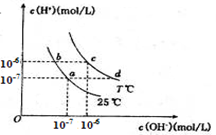
| A��a���Ӧ����ɫ��Һ���ܴ������ڣ�Fe3+��Na+��Cl-��SO42- |
| B��b���Ӧ����Һ���ܴ������ڣ�NH4+��Ca2+��AlO2-��I- |
| C��c���Ӧ����Һ���ܴ������ڣ�Na+��Ba2+��Cl-��CO32- |
| D��d���Ӧ����Һ���ܴ������ڣ�Na+��K+��SO32-��Cl- |
����������Һ���ܴ������棬ͨ��SO2�����ܴ��������һ����
| A��Ba2+��Na+��H+��Cl�� | B��Na+��K+��SO32����Cl�� |
| C��Al3+��K+��Br����HCO3�� | D��Fe3+��Na+��H2O2��SO42�� |
�����£����и���������ָ����Һ���ܴ���������� �� ��
A�������̪�Ժ�ɫ����Һ��Na+��Al3+��SO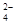 ��Cl�� ��Cl�� |
B������KSCN�Ժ�ɫ����Һ��Na+��Cu2+��Br����SO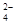 |
C��c( Fe2+)= 1mol��L��l����Һ��H+��Na+��Cl����NO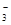 |
D�����������ܲ�������H2����Һ��Na+��K+��Cl����HCO |
������������Һ���ܴ������棬ͨ��SO2��������ܴ��������һ����
| A��NH4+��I����Cl����Ca2+ | B��K+��Na+��ClO����SO42�� |
| C��Fe3+��Na+��Cl����SCN�� | D��K+��Al3+��NO3����AlO2�� |
�����£����и���������ָ����Һ��һ���ܴ����������
| A��0.1mo1/L NaI��Һ��K+��Na+��MnO4-��OH- |
| B�����ܽ�CaCO3����Һ��K+��NH4+��Cl-��NO3- |
| C��0.1mo1/LNaHSO3��Һ��Na +��Mg2+��SO42-��ClO- |
| D��c(H+)/c(OH-)=1013������Һ��K+��Fe2+��Cl-��NO3- |
������������ˮ��Һ�д��������һ����
| A��Fe3+��HCO3����Cl����SCN�� | B��Ba2+��NO3����SO32����H+ |
| C��Mg2+��NH4+��Br����OH�� | D��Na+��Cu2+��SO42����Cl�� |