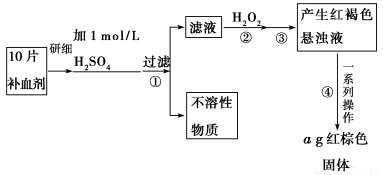
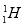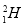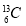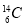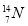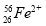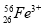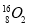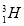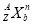��Ŀ����
�Ȼ���ͭ(CuCl)�ǰ�ɫ��ĩ��������ˮ���Ҵ���ϡ���ᣬ�۵�422
�����е�1366 �����ڿ�����Ѹ�ٱ���������ɫ���������л��ϳɹ�ҵ�еĴ������Դ���ˮ(��Ca2����Mg2����SO42��������)��Cu��ϡ���ᡢSO2��Ϊԭ�Ϻϳ�CuCl�Ĺ����������£�
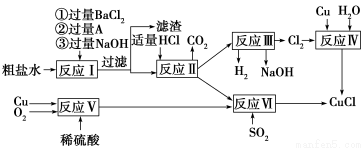
(1)A�Ļ�ѧʽΪ________��
(2)д����Ӧ���Ļ�ѧ����ʽ______________________________________
(3)д����Ӧ�������ӷ���ʽ______________________________________
(4)�������п���ѭ�����õ�������(�û�ѧʽ��ʾ)___________________________
(5)��Ӧ�����˵õ�CuCl����������ˮ�Ҵ�ϴ�ӳ���������ո��������70 ����2Сʱ����ȴ���ܷ��װ���ò�Ʒ����70 ����ո����Ŀ����_________________________
(1)Na2CO3
(2)2Cu��O2��2H2SO4=2CuSO4��2H2O
(3)2Cu2����2Cl����SO2��2H2O=2CuCl����4H����SO42��
(4)NaOH��H2SO4
(5)�ӿ��Ҵ���ˮ����������ֹCuCl����������
���������Ʊ����̻��漰�������ᴿ��ʵ�飬Ҫע���������ۺϿ��ǡ�Ϊ��ʹ���ʾ���������һ�����ij����Լ���Ҫ�����������ij����Լ��ں�ߵIJ����б���ȥ��

��ν�Ͻ𣬾��Dz�ͬ�ֽ���(Ҳ����һЩ�ǽ���)���ۻ�״̬���γɵ�һ���ۺ���±�Ϊ���ֽ������ۡ��е㣺
| Na | Cu | Al | Fe |
�۵�(��) | 97.5 | 1 083 | 660 | 1 535 |
�е�(��) | 883 | 2 595 | 2 200 | 3 000 |
�������������ж����в����γɺϽ����(����)
A��Cu��Na������������B��Fe��Cu
C��Fe��Al D��Al��Na
�ס��ҡ��������ֲ�����ͬ���ӵĿ�����ǿ����ʡ������������������±���ʾ��
������ | NH4+��Na����Mg2�� |
������ | OH����NO3����SO42�� |
ȡ�����������ֻ��������Ƴ���ͬ�������Һ�������ʵ����ʵ���Ũ�ȣ�c(��)��c(��)��c(��)��
(1)����________����AlCl3��Һ����μ������Һ���۲쵽��������________����Ӧ�����ӷ���ʽΪ________��
(2)����______�����ʵ��ȷ���ҵ��������________(������ȷ��������ÿ�)��