��Ŀ����
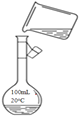
����Ŀ��ʵ��������ͼװ����ȡ�����屽���ش��������⡣

��1��������ƿ�з�Ӧ�Ļ�ѧ����ʽ�ǣ�__________________________________��
��2�����뱽��Һ�壬�۲쵽������ƿ�е�����_____________��
��3����Ӧ����������ƿ���ܳ��ڸ������Թ۲쵽�������� ___________________�����ܿڲ��ܲ�����ƿ��Һ�����µ�ԭ����__________________________________��
��4��ϴ��ƿ��ʢ��CCl4��������_____________������ܵ������� ________________��
��5����֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ��������ƿ�м���AgNO3��Һ������������ɫ����������֤������һ����֤�ķ���������ƿ�м���_________��������__________��
���𰸡�C6H6 + Br2 ![]() C6H5Br + HBr��ӦҺ�У��к���ɫ�������������ƿ���������廯�⼫������ˮ����ֹ������ȥ�廯�������е�������β������ʯ����Һ��Һ���ɫ
C6H5Br + HBr��ӦҺ�У��к���ɫ�������������ƿ���������廯�⼫������ˮ����ֹ������ȥ�廯�������е�������β������ʯ����Һ��Һ���ɫ
��������
(1)������ƿ�б���Һ��������������ʱ���������屽���廯�⣬��Ӧ�Ļ�ѧ����ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(2)����(1)�ķ��������뱽��Һ����������ƿ�еķ�ӦҺ�У��к���ɫ�������������ƿ���ʴ�Ϊ����ӦҺ�У��к���ɫ�������������ƿ��
(3)��Ӧ����������ƿ���ܳ��ڸ������������СҺ�Σ���˿��Թ۲쵽�������Dz����������廯�⼫������ˮ��Ϊ�˷�ֹ���������ܿڲ��ܲ�����ƿ��Һ�����£��ʴ�Ϊ�������������廯�⼫������ˮ����ֹ������
(4)��������ƿ�����������к������������廯�⣬�������������������Ȼ�̼��ϴ��ƿ��ʢ��CCl4���Գ�ȥ�廯�������е���������������еļ�ʯ�ҿ�������β���е��廯�⣬��ֹ��Ⱦ���ʴ�Ϊ����ȥ�廯�������е���������β�����գ�
(5)��֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ��������ƿ�м���AgNO3��Һ������������ɫ������˵���������廯�⣬��˿���֤������Һ�巢������ȡ����Ӧ��֤������Һ�巢������ȡ����Ӧ��ֻ��Ҫ֤�����廯�����ɣ������һ����֤�ķ���������ƿ�м���ʯ����Һ������Һ���ɫ������֤�����ʴ�Ϊ��ʯ����Һ����Һ���ɫ��

����Ŀ������ѡ���У�Ϊ�����Ӧʵ�飬������������ز����������ǣ� ��
A | B | C | D |
����1mol/L AlCl3��Һ | ʵ������ȡ ���� | �Ƚ���̼�������� Ԫ�صķǽ�����ǿ�� | ʢװNaOH ��Һ |
|
|
|
|
A. AB. BC. CD. D