��Ŀ����
ijУ������ѧ�о���ѧϰС�飬��ѧϰ��ͭ��Ũ����ķ�Ӧ��ֱ�̽��������п��Ũ���ᷴӦ�Ĺ��̡���С���������ͼװ�ã��Իش�
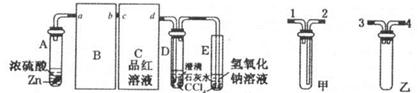
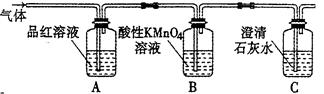
(1)��Ӽס�����ѡ����ʵ�װ������B��C�У���������ȷ���ӣ�a��______, c��______(����ű�ʾ����
(2)D��E��֧�Թ���CCl4��������______��
(3)��ʵ����֤��Ũ�������ǿ�����Ե�ʵ������Ϊ______��
(4)D�г��ֻ��ǵ����ӷ���ʽΪ______��
(5)ijѧ��ע��۲쵽��ʵ�鿪ʼ��C��D��E�о������ݲ�����������������٣�Ʒ����Һ��ɫ��D�г��ֻ��ǣ���Ӧһ��ʱ���C��D��E�е��������ֻ��������ӡ����û�ѧ����ʽ��ʾ����Ӧһ��ʱ����������ֻ��������ӡ���ԭ����______��
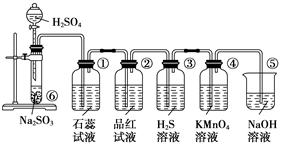
��С���������ͼװ�ã�
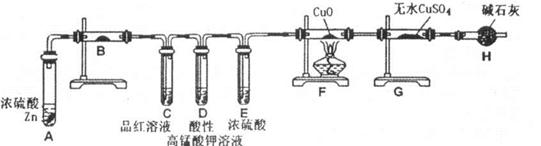
(6)��������С��������ͼ��______��
(7)װ��B�е�ҩƷ��______��
(8)��֤������SO2��H2�Ⱥ�˳���ʵ��������______��

(1)��Ӽס�����ѡ����ʵ�װ������B��C�У���������ȷ���ӣ�a��______, c��______(����ű�ʾ����
(2)D��E��֧�Թ���CCl4��������______��
(3)��ʵ����֤��Ũ�������ǿ�����Ե�ʵ������Ϊ______��
(4)D�г��ֻ��ǵ����ӷ���ʽΪ______��
(5)ijѧ��ע��۲쵽��ʵ�鿪ʼ��C��D��E�о������ݲ�����������������٣�Ʒ����Һ��ɫ��D�г��ֻ��ǣ���Ӧһ��ʱ���C��D��E�е��������ֻ��������ӡ����û�ѧ����ʽ��ʾ����Ӧһ��ʱ����������ֻ��������ӡ���ԭ����______��
��С���������ͼװ�ã�

(6)��������С��������ͼ��______��
(7)װ��B�е�ҩƷ��______��
(8)��֤������SO2��H2�Ⱥ�˳���ʵ��������______��
��15�֣�(1) 3(��4) 2����1�֣���2�֣� (2) ��������2�֣�
(3) C��Ʒ����Һ��ɫ��2�֣�
(4) Ca2+��2OH-��SO2===CaSO3��+H2O��2�֣�
(5) Zn+H2SO4��ϡ���� ZnSO4+H2����2�֣�
(6) ��֤�������Ȳ���ˮ��SO2�������H2��2�֣����������𰸲��ո��֣�
(7) ��ˮCuSO4(����ˮ����ͭ) ��1�֣�
(8) C��Ʒ����ɫ��D�����Ը��������ɫ���ٱ�dz��F�й����ɺ�ɫ���ɫ��G����ˮ����ͭ������2�֣����������𰸲��ո��֣�
(3) C��Ʒ����Һ��ɫ��2�֣�
(4) Ca2+��2OH-��SO2===CaSO3��+H2O��2�֣�
(5) Zn+H2SO4��ϡ���� ZnSO4+H2����2�֣�
(6) ��֤�������Ȳ���ˮ��SO2�������H2��2�֣����������𰸲��ո��֣�
(7) ��ˮCuSO4(����ˮ����ͭ) ��1�֣�
(8) C��Ʒ����ɫ��D�����Ը��������ɫ���ٱ�dz��F�й����ɺ�ɫ���ɫ��G����ˮ����ͭ������2�֣����������𰸲��ո��֣�
�����������1������ͭ��Ũ���ᷴӦ�ķ���ʽ��֪��п��Ũ���ᷴӦ�ķ���ʽΪZn+2H2SO4��Ũ���TZnSO4+SO2��+2H2O������ͨ��״���£�1���ˮ�п��ܽ�40����Ķ����������壬���װ���ҿɷ�ֹ����������Ʒ������ʱ������п�����ᷴӦ�������У�����������Ʒ�����ã��賤�ܽ���������ȷ������˳����3����4����4����3����b��c��2��1��d��
��2����������������ˮ�����������Ȼ�̼����������ͨ�����Ȼ�̼��Ȼ����������ʯ��ˮ��Ӧ�������ã��ɷ�ֹ������
��3�����������ʹƷ����Һ��ɫ�������ڼ����������Ĵ��ڣ����Ա�ʵ����֤��Ũ�������ǿ�����Ե�ʵ������ΪC��Ʒ����Һ��ɫ��
��4����������ͳ����ʯ��ˮ��Ӧ����������ƺ�ˮ���������Ϊ������ˮ�ij�������Ӧ�����ӷ���ʽΪCa2++2OH-+SO2�TCaSO3��+H2O��
��5��пΪ�ϻ��õĽ������ڽ����˳������������ǰ�棬����п�ܺ�ϡ���ᷴӦ��������п�������������ŷ�Ӧ�Ľ��У�Ũ�����Ũ�����ͣ�����Ϊϡ�������п��Ӧ������������Ӧ�Ļ�ѧ����ʽΪZn+H2SO4��ϡ���TZnSO4+H2����
��6������װ��ͼ��֪����С����Ƶ�װ�ã����ð�ɫ����ˮ����ͭ����ˮ�Ĵ��ڣ�����Ʒ������������Ĵ��ڣ��ø�������������������ȥ��������Ũ���������ˮ�ԣ���ȥˮ��п��ϡ���ᷴӦ��������������п������ͨ���ȵ�����ͭ����ͭ��ˮ��ˮ��������ˮ����ͭ����ɫ���ɼ��������Ĵ��ڣ���˸���Ƶ�Ŀ������֤�������Ȳ���ˮ��SO2�������H2��
��7����������ͨ����Һʱһ�������ˮ����������Ҫ���ȼ���ˮ����������ˮ��������ˮ����ͭ����ɫ��֪��װ��B�е�ҩƷ����ˮCuSO4������ˮ����ͭ������������ˮ�Ĵ��ڡ�
��8�����������ʹƷ����Һ��ɫ��C��Ʒ����ɫ���ɼ����������Ĵ��ڡ�SO2�����л�ԭ�ԣ��������������������ɳ�ȥ��������D�����Ը�����ز�����ɫ��˵�����������Ѿ�������п��ϡ���ᷴӦ��������������п������ͨ���ȵ�����ͭ����ͭ��ˮ��F���к�ɫ�������ɣ�˵����ͭ���ɣ�G����ˮ����ͭ������˵����ˮ���ɣ���֤��������������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
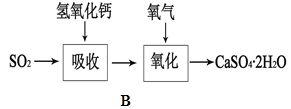
 CO2��+2SO2��+2H2O������ŨH2 S04����������� �������������ԭ������������0��2mol̼����ȫ��Ӧ��������H2S04�������� g������²���SO2�����Ϊ______________L��
CO2��+2SO2��+2H2O������ŨH2 S04����������� �������������ԭ������������0��2mol̼����ȫ��Ӧ��������H2S04�������� g������²���SO2�����Ϊ______________L��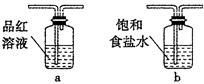
 [
[