��Ŀ����
����Ŀ��ʵ������ȡ����ͨ�������ַ�����
���ù��������������Ȼ�粒��ȣ�
���ڳ������ù�������������Ũ��ˮ��Ӧ��
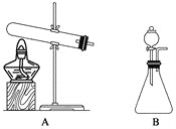
(1)�������ȡװ��ͼ�У�������Ӧѡ��װ��__________(����A������B������ͬ)��������Ӧѡ��װ��__________��
(2)�����Ȼ�����������ƻ������ȡ�����ķ�Ӧ����ʽ��________________��
(3)����ȡ�����Ҫ���ﰱ����Ӧѡ�õĸ������________________������ĸ����
A��Ũ���ᡡ����B����ʯ�ҡ�����C������������
(4)���鼯��ƿ���Ƿ��ռ��������ķ�����__________________________��
���𰸡�A B 2NH4Cl��Ca(OH)2![]() CaCl2��2NH3����2H2O B ��ʪ��ĺ�ɫʯ����ֽ��������ƿ�ڣ�����ֽ������˵�������Ѽ���
CaCl2��2NH3����2H2O B ��ʪ��ĺ�ɫʯ����ֽ��������ƿ�ڣ�����ֽ������˵�������Ѽ���
��������
(1)�ù��������������Ȼ�粒����Ʊ��������ǹ����������Ʊ�����ķ�Ӧ���ڳ������ù�������������Ũ��ˮ��Ӧ�ǹ����Һ�岻�����Ʊ����壻
(2)��ȡNH3�ķ�ӦΪ����NH4Cl��Ca(OH)2��Ӧ�����Ȼ��ơ�������ˮ��
(3)�����Ǽ������壬���������Ը�������Ӧ�ü��Ը������
(4)�����Ǽ������壬����ʪ��ĺ�ɫʯ����ֽ����ɫ��
(1)�ù��������������Ȼ�粒����Ʊ��������ǹ����������Ʊ�����ķ�Ӧ��Ӧѡ��Aװ�ã��ڳ������ù�������������Ũ��ˮ��Ӧ�ǹ����Һ�岻�����Ʊ�������Ӧѡ��Bװ�ã�
(2)��ȡNH3�ķ�ӦΪ����NH4Cl��Ca(OH)2��Ӧ�����Ȼ��ơ�������ˮ����Ӧ�Ļ�ѧ����ʽΪ��2NH4Cl+Ca(OH)2 ![]() CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O��
(3)A��Ũ����Ͱ����ᷴӦ��������泥����ܸ��ﰱ����A����
B�������������ƾ�����ʪ�ԣ����Ը��ﰱ����B��ȷ��
C�����������Ͱ���������Ӧ�����ܸ��ﰱ����C����
�ʺ���ѡ����B��
(4)��ʪ��ĺ�ɫʯ����ֽ��������ƿ�ڣ������ֽ��������֤���ǰ����Ѿ��ռ�����