��Ŀ����
���мס��ҡ�������4�����ʣ���ת����ϵ���£�
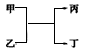
��ش�
(1)��X��Y��Z��ԭ���������������3�ֶ�����Ԫ�أ�X��ԭ�Ӱ뾶��С��Ԫ�أ�YԪ��ԭ�ӵ������������Ǵ�����������3����
��YԪ�������ڱ��е�λ����___��
�ڵ���ΪZԪ����ɵĵ��ʣ���(X2Y)��������ʱ��д�������¼����ҷ�Ӧ�����ӷ���ʽ:___��
(2)����Ϊ��ʹʪ��ĺ�ɫʯ����ֽ���������壻��Ϊ���ʣ������Ԫ�ص�ԭ�Ӻ�������������Է���������ֵ��ȣ���Ϊʵ������ȡ�ij����Լ�����Ϊ�����к����������塣
�����й��ڱ���˵����ȷ����___������ĸ����
a�����ȶ��������ֽ�
b��������ֻ���й��ۼ�
c���������ַǽ���Ԫ����ɵ���
d��ʵ���ҳ��������������ƹ�����ȡ��
��������5.6 g������Ӧ������ת�Ƶ�������Ϊ____mol��
(1)��X��Y��Z��ԭ���������������3�ֶ�����Ԫ�أ�X��ԭ�Ӱ뾶��С��Ԫ�أ�YԪ��ԭ�ӵ������������Ǵ�����������3����
��YԪ�������ڱ��е�λ����___��
�ڵ���ΪZԪ����ɵĵ��ʣ���(X2Y)��������ʱ��д�������¼����ҷ�Ӧ�����ӷ���ʽ:___��
(2)����Ϊ��ʹʪ��ĺ�ɫʯ����ֽ���������壻��Ϊ���ʣ������Ԫ�ص�ԭ�Ӻ�������������Է���������ֵ��ȣ���Ϊʵ������ȡ�ij����Լ�����Ϊ�����к����������塣
�����й��ڱ���˵����ȷ����___������ĸ����
a�����ȶ��������ֽ�
b��������ֻ���й��ۼ�
c���������ַǽ���Ԫ����ɵ���
d��ʵ���ҳ��������������ƹ�����ȡ��
��������5.6 g������Ӧ������ת�Ƶ�������Ϊ____mol��
(1)�ٵڶ����ڵڢ�A�壻 ��2Na+2H2O==2Na++2OH-+H2��
(2)��ac����1.2
(2)��ac����1.2

��ϰ��ϵ�д�
�����Ŀ

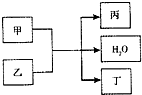 ���мס��ҡ�������4�����ʣ����м�Ϊ���ʣ���������Ϊ�����ת����ϵ���£�
���мס��ҡ�������4�����ʣ����м�Ϊ���ʣ���������Ϊ�����ת����ϵ���£�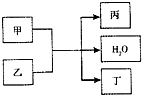 ���мס��ҡ�������4�����ʣ����м�Ϊ���ʣ���������Ϊ�����ת����ϵ���£�
���мס��ҡ�������4�����ʣ����м�Ϊ���ʣ���������Ϊ�����ת����ϵ���£�