��Ŀ����
���мס��ҡ��������������ʣ��乹����������14�����ӣ���֪���ǹ��嵥�ʣ�����ֻ���Ǽ��Թ��ۼ���������ӣ�������ԭ�ӷ��ӣ�����һ�����ӻ�����������Ӱ뾶���������Ӱ뾶��
��1�����γɵľ������� ��������ͣ��������ӵĿռ乹���� ��
��2�����ĵ���ʽΪ ��
��3����֪����ȼ����ΪakJ?mol-1����д����ȼ�շ�Ӧ���Ȼ�ѧ����ʽ ��
��4�����ʼס��Ҵ������ϵ��ͼ������F��һ�ֳ���������

��д�����з���ʽ��
�ټ���NaOH��Һ�����ӷ�Ӧ�� ��
�����뵥��F�Ļ�ѧ��Ӧ�� ��
��1�����γɵľ�������
��2�����ĵ���ʽΪ
��3����֪����ȼ����ΪakJ?mol-1����д����ȼ�շ�Ӧ���Ȼ�ѧ����ʽ
��4�����ʼס��Ҵ������ϵ��ͼ������F��һ�ֳ���������

��д�����з���ʽ��
�ټ���NaOH��Һ�����ӷ�Ӧ��
�����뵥��F�Ļ�ѧ��Ӧ��
�������ס��ҡ��������������ʣ��乹����������14�����ӣ����ת����ϵ���֪�����ǹ��嵥�ʣ���NaOH��Һ��Ӧ�����ΪSi������ֻ���Ǽ��Թ��ۼ���������ӣ���ΪN2��FΪMg��������ԭ�ӷ��ӣ���ΪC2H2������һ�����ӻ�����������Ӱ뾶���������Ӱ뾶����ΪLi2O��Ȼ�������ʵ����ʼ���ѧ���������
����⣺�ס��ҡ��������������ʣ��乹����������14�����ӣ����ת����ϵ���֪�����ǹ��嵥�ʣ���NaOH��Һ��Ӧ�����ΪSi������ֻ���Ǽ��Թ��ۼ���������ӣ���ΪN2��FΪMg��������ԭ�ӷ��ӣ���ΪC2H2������һ�����ӻ�����������Ӱ뾶���������Ӱ뾶����ΪLi2O��
��1��SiΪԭ�Ӿ��壬��ȲΪֱ���ͷ��ӣ��ʴ�Ϊ��ԭ�Ӿ��壻ֱ���ͣ�
��2����ΪLi2O�������ʽΪ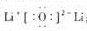 ���ʴ�Ϊ��
���ʴ�Ϊ��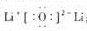 ��
��
��3������ȼ����ΪakJ?mol-1����ȼ�շ�Ӧ���Ȼ�ѧ����ʽΪ2C2H2��g��+5O2��g��=4CO2��g��+2H2O��l����H=-2akJ/mol��
�ʴ�Ϊ��2C2H2��g��+5O2��g��=4CO2��g��+2H2O��l����H=-2akJ/mol��
��4���ټ���NaOH��Һ�����ӷ�ӦΪSi+2OH-+H2O=SiO32-+2H2�����ʴ�Ϊ��Si+2OH-+H2O=SiO32-+2H2����
�����뵥��F�Ļ�ѧ��ӦΪ3Mg+N2
Mg3N2���ʴ�Ϊ��3Mg+N2
Mg3N2��
��1��SiΪԭ�Ӿ��壬��ȲΪֱ���ͷ��ӣ��ʴ�Ϊ��ԭ�Ӿ��壻ֱ���ͣ�
��2����ΪLi2O�������ʽΪ
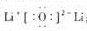 ���ʴ�Ϊ��
���ʴ�Ϊ��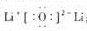 ��
����3������ȼ����ΪakJ?mol-1����ȼ�շ�Ӧ���Ȼ�ѧ����ʽΪ2C2H2��g��+5O2��g��=4CO2��g��+2H2O��l����H=-2akJ/mol��
�ʴ�Ϊ��2C2H2��g��+5O2��g��=4CO2��g��+2H2O��l����H=-2akJ/mol��
��4���ټ���NaOH��Һ�����ӷ�ӦΪSi+2OH-+H2O=SiO32-+2H2�����ʴ�Ϊ��Si+2OH-+H2O=SiO32-+2H2����
�����뵥��F�Ļ�ѧ��ӦΪ3Mg+N2
| ||
| ||
���������⿼��������ƶϣ��������ʵ���������������14���������ƶ�����Ϊ���Ĺؼ����漰����ʽ��ȼ���ȡ����ӷ�Ӧ�Ŀ��飬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
���мס��ҡ���������֧�Թܣ��ס����о�ʢ��1mL������KMnO4��Һ���ҡ����зֱ�ʢ��2mL��ˮ+2g FeBr3��2mL Br2��CCl4��Һ+2g FeBr3������ס����м������������������м��������ױ���������ã������йط�������ȷ��һ���ǣ�������
|