��Ŀ����
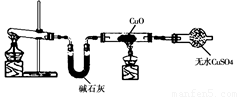
ijѧϰС�鰴��ͼ��ʵ������ȡ������̽��ͭ���й����ʣ����ּг�����δ����������ش�
��1����ȡ�����Ļ�ѧ����ʽ�� ��
��2���� ʵ������Ϊ����ɫCuO��Ϊ��ɫ�����ɵ���a������ɫ��ˮCuSO4��ĩ��Ϊ��ɫ�� ͬʱ����һ����ɫ���壬����������Ⱦ����д��������CuO��Ӧ�Ļ�ѧ����ʽ ��
�ڼ�ʯ�ҵ������� ��
��3�����������ɵĵ���a����ˮԡ�н���4��ʵ�飬����ʵ�鱨�����±���ʾ��
|
��� |
ʵ����� |
ʵ������ |
|
�� |
ϡ�����м���õ���a |
�����Ա仯 |
|
�� |
��������Һ�м���õ���a |
�����Ա仯 |
|
�� |
��������Һ�м���õ���a |
�����Ա仯 |
|
�� |
ϡ�����м�����������Һ |
�����Ա仯 |
|
�ټ���õ���a |
����ɫ���ݣ���Һ���� |
��ʵ��I��II��III��Ŀ���� ��
��ʵ����з�Ӧ�ı����ǣ������ӷ���ʽ��ʾ�� ��
(ÿ��2�֣���10��) ��1��Ca��OH��2��2NH4Cl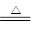 CaCl2��2H2O��2NH3��
CaCl2��2H2O��2NH3��
��2����3CuO +2NH3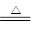 3Cu+N2+3H2O �����հ����л��е�ˮ��������ֹ���Ų���ļ�����
3Cu+N2+3H2O �����հ����л��е�ˮ��������ֹ���Ų���ļ�����
��3����˵�������ӡ���������ӡ���������Ӿ����ܵ���ʹͭ�ܽ�
��3Cu��2NO3����8H+��3Cu2+��2NO����4H2O
��������
�����������1����ȡ�����Ļ�ѧ����ʽ��Ca��OH��2��2NH4Cl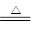 CaCl2��2H2O��2NH3����
CaCl2��2H2O��2NH3����
��2���ٺ�ɫCuO��Ϊ��ɫ�����ɵ���a������a��ͭ����ɫ��ˮCuSO4��ĩ��Ϊ��ɫ����˵����Ӧ����ˮ���ɡ�ͬʱ����һ����ɫ���壬����������Ⱦ�������ԭ���غ��֪���������ǵ�������������CuO��Ӧ�Ļ�ѧ����ʽ��3CuO
+2NH3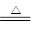 3Cu+N2+3H2O��
3Cu+N2+3H2O��
���������ɵİ����к���ˮ��������ˮ���������ʵ�飬��˼�ʯ�ҵ����������հ����л��е�ˮ��������ֹ���Ų���ļ�����
��3���ٸ���ʵ����̿�֪��ʵ��I��II��III��ʵ����Ķ���ʵ�飬����ʵ��I��II��III��Ŀ����˵�������ӡ���������ӡ���������Ӿ����ܵ���ʹͭ�ܽ⡣
�ڸ���ʵ�������֪�������������£�����������������ͭ����Ӧ�����ӷ���ʽ��3Cu��2NO3����8H+��3Cu2+��2NO����4H2O��
���㣺���鰱�����Ʊ����������ʵļ����Լ�������ԭ��Ӧ���й��жϺ�Ӧ��
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ�����ض�ѧ��ʵ�������������ͽ��ⷽ����ָ��������������ѧ���淶���Ͻ���ʵ����ƺ���������������������Ҫ����ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ�������������

ijѧϰС�鰴��ͼ��ʵ������ȡ������̽��ͭ���й����ʣ����ּг�����δ��������
��ش�
��1����ȡ�����Ļ�ѧ����ʽ�� ��
��2���� ʵ������Ϊ����ɫCuO��Ϊ��ɫ�����ɵ���a������ɫ��ˮCuSO4��ĩ��Ϊ��ɫ�� ͬʱ����һ����ɫ���壬����������Ⱦ����д��������CuO��Ӧ�Ļ�ѧ����ʽ �� �ڼ�ʯ�ҵ������� ��
��3�����������ɵĵ���a����ˮԡ�н���4��ʵ�飬����ʵ�鱨�����±���ʾ��
| ��� | ʵ����� | ʵ������ |
| �� | ϡ�����м���õ���a | �����Ա仯 |
| �� | ��������Һ�м���õ���a | �����Ա仯 |
| �� | ��������Һ�м���õ���a | �����Ա仯 |
| �� | ϡ�����м�����������Һ | �����Ա仯 |
| �ټ���õ���a | ����ɫ���ݣ���Һ���� |
��ʵ����з�Ӧ�ı����ǣ������ӷ���ʽ��ʾ�� ��
�۽��õ���a���뵽��������Ĺ���������Һ�У���Һ�����ɫ���÷�Ӧ�����ӷ���ʽ�� ��
ijѧϰС�鰴��ͼ��ʵ������ȡ������̽��ͭ���й����ʣ����ּг�����δ����������ش�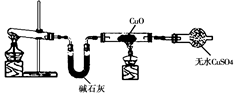
��1����ȡ�����Ļ�ѧ����ʽ�� ��
��2���� ʵ������Ϊ����ɫCuO��Ϊ��ɫ�����ɵ���a������ɫ��ˮCuSO4��ĩ��Ϊ��ɫ�� ͬʱ����һ����ɫ���壬����������Ⱦ����д��������CuO��Ӧ�Ļ�ѧ����ʽ ��
�ڼ�ʯ�ҵ������� ��
��3�����������ɵĵ���a����ˮԡ�н���4��ʵ�飬����ʵ�鱨�����±���ʾ��
| ��� | ʵ����� | ʵ������ |
| �� | ϡ�����м���õ���a | �����Ա仯 |
| �� | ��������Һ�м���õ���a | �����Ա仯 |
| �� | ��������Һ�м���õ���a | �����Ա仯 |
| �� | ϡ�����м�����������Һ | �����Ա仯 |
| �ټ���õ���a | ����ɫ���ݣ���Һ���� |
��ʵ����з�Ӧ�ı����ǣ������ӷ���ʽ��ʾ�� ��
ijѧϰС�鰴��ͼ��ʵ������ȡ������̽��ͭ���й����ʣ����ּг�����δ��������
��ش�

��1����ȡ�����Ļ�ѧ����ʽ�� ��
��2���� ʵ������Ϊ����ɫCuO��Ϊ��ɫ�����ɵ���a������ɫ��ˮCuSO4��ĩ��Ϊ��ɫ�� ͬʱ����һ����ɫ���壬����������Ⱦ����д��������CuO��Ӧ�Ļ�ѧ����ʽ �� �ڼ�ʯ�ҵ������� ��
��3�����������ɵĵ���a����ˮԡ�н���4��ʵ�飬����ʵ�鱨�����±���ʾ��
|
��� |
ʵ����� |
ʵ������ |
|
�� |
ϡ�����м���õ���a |
�����Ա仯 |
|
�� |
��������Һ�м���õ���a |
�����Ա仯 |
|
�� |
��������Һ�м���õ���a |
�����Ա仯 |
|
�� |
ϡ�����м�����������Һ |
�����Ա仯 |
|
�ټ���õ���a |
����ɫ���ݣ���Һ���� |
��ʵ��I��II��III��Ŀ���� ��
��ʵ����з�Ӧ�ı����ǣ������ӷ���ʽ��ʾ�� ��
�۽��õ���a���뵽��������Ĺ���������Һ�У���Һ�����ɫ���÷�Ӧ�����ӷ���ʽ�� ��
ijѧϰС�鰴��ͼ��ʵ������ȡ������̽��ͭ���й����ʣ����ּг�����δ��������
��ش�
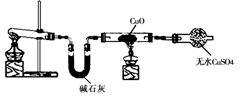
��1����ȡ�����Ļ�ѧ����ʽ��
�������������������������� ��
��2���� ʵ������Ϊ����ɫCuO��Ϊ��ɫ
�����ɵ���a������ɫ��ˮCuSO4��ĩ
��Ϊ��ɫ�� ͬʱ����һ����ɫ���壬
����������Ⱦ��
��д��������CuO��Ӧ�Ļ�ѧ����ʽ���������� ����
�ڼ�ʯ�ҵ��������������������� ��
��3�����������ɵĵ���a����ˮԡ�н���4��ʵ�飬����ʵ�鱨�����±���ʾ��
| ��� | ʵ����� | ʵ������ |
| �� | ϡ�����м���õ���a | �����Ա仯 |
| �� | ��������Һ�м���õ���a | �����Ա仯 |
| �� | ��������Һ�м���õ���a | �����Ա仯 |
| �� | ϡ�����м�����������Һ | �����Ա仯 |
| �ټ���õ���a | ����ɫ���ݣ���Һ���� |
��ʵ��I��II��III��Ŀ�������������������� ��
��ʵ����з�Ӧ�ı����ǣ������ӷ���ʽ��ʾ���������������� ������������ ��
�۽��õ���a���뵽��������Ĺ���������Һ�У���Һ�����ɫ���÷�Ӧ�����ӷ���ʽ������������������ ��