��Ŀ����
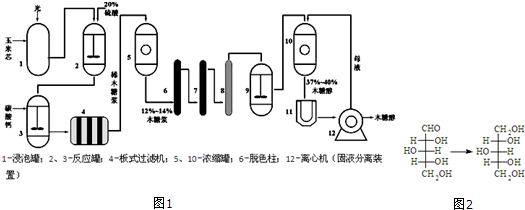
8��ľ�Ǵ���C5H12O5����������ζ����Ӫ�������ڻ�����ʳƷ��ҽҩ�ȹ�ҵ���й㷺Ӧ�ã���������о�еĶ��ǿ�������ľ�Ǵ����乤��������ͼ1����֪��ľ����ľ�Ǵ���ת����ϵ��ͼ2��ʾ��
��ش��������⣺
��1��װ��2���������Ҫ�����Ǵ���
��2��װ��3�м���̼��Ƶ�Ŀ�����к�ʣ������ᣬ��������������ڷ��룮
��3������ɫ����������������ǻ���̿��Ϊ��ȥľ�ǽ��е��������ӣ�7��8װ���е�����������������ӽ���Ĥ�������ӽ���Ĥ��
��4��װ��9��������D��A����ȴľ�ǽ�B��ˮ��ľ��C������ľ��D����ԭľ��
��5��װ��11�������ǵõ�ľ�Ǵ����壬�ò����������ǽᾧ���õ���ľ�Ǵ������к���2������̼ԭ�ӣ�
���� ����������������о�еĶ�������ľ�Ǵ������̿�֪������о������������ˮ���ľ�ǽ���ľ�ǽ�������̼��ơ����ӽ���Ĥ�ȹ��̳��ӣ��ýϴ���ľ�ǽ���ľ�ǽ��ٻ�ԭ��ľ�Ǵ���Ȼ��ͨ���ᾧ��ľ�Ǵ���
��1���������������������������·���ˮ�⣻
��2��װ��2�е���������װ��3�У�����̼��ƿ��Գ�ȥ������е����
��3������̼���������ã���������ɫ����ľ�ǽ��е��������ӣ�����ͨ�������ӽ���Ĥ�������ӽ���Ĥ��ȥ��
��4��������ˮ��õ�����ľ��Ϊ���ǻ�ȩ��ͨ��װ��9�ɽ�ȩ����ԭ���ǻ���
��5��װ��11�������ǽ�37%-40%ľ�Ǵ����õ�ľ�Ǵ�������ͨ���ᾧ�ķ���������̼ԭ����Χ���в�ͬ��ԭ�ӻ�ԭ���ţ�����ͼ2��ľ�Ǵ��Ľṹ��ʽ���⣮
��� �⣺����������������о�еĶ�������ľ�Ǵ������̿�֪������о������������ˮ���ľ�ǽ���ľ�ǽ�������̼��ơ����ӽ���Ĥ�ȹ��̳��ӣ��ýϴ���ľ�ǽ���ľ�ǽ��ٻ�ԭ��ľ�Ǵ���Ȼ��ͨ���ᾧ��ľ�Ǵ���
��1���������������������������·���ˮ�⣬
�ʴ�Ϊ������
��2��װ��2�е���������װ��3�У�����̼��ƿ��Գ�ȥ������е����ᣬ��������������ڷ��룬
�ʴ�Ϊ���к�ʣ������ᣬ��������������ڷ��룻
��3������̼���������ã���������ɫ������������ɫ����������������ǻ���̿��ľ�ǽ��е��������ӣ�����ͨ�������ӽ���Ĥ�������ӽ���Ĥ��ȥ��
�ʴ�Ϊ������̿�������ӽ���Ĥ��
��4��������ˮ��õ�����ľ��Ϊ���ǻ�ȩ��ͨ��װ��9�ɽ�ȩ����ԭ���ǻ�����ѡD��
��5��װ��11�������ǽ�37%-40%ľ�Ǵ����õ�ľ�Ǵ�������ͨ���ᾧ�ķ���������̼ԭ����Χ���в�ͬ��ԭ�ӻ�ԭ���ţ�����ͼ2��ľ�Ǵ��Ľṹ��ʽ��֪����������2������̼��
�ʴ�Ϊ���ᾧ��2��
���� ������Ҫ����ľ�Ǵ����Ʊ����е��Ѷȣ�����ʱע����Ʊ�ԭ����ҵ���̵����⣮

 ��У����ϵ�д�
��У����ϵ�д�| A�� | MgCl2 Na2SO4 BaCl2 KOH | |
| B�� | BaCl2 AgNO3 CaCl2 HNO3 | |
| C�� | NaOH Na2SO4 KNO3 HCl | |
| D�� | HCl Na2CO3 Na2SO4 BaCl2 |
 1-��������[��CH3��2CHCH2CH2Br]���л��ϳɵ���Ҫ�м��壬����������Ⱦ�ϡ������ȣ���е�Ϊ121�棬������CCl4���������촼��������������������·�Ӧ���ã���CH3��2CHCH2CH2OH+HBr$\stackrel{��}{��}$��CH3��2CHCH2CH2Br+H2O����֪���촼�ķе�Ϊ132.5�棬����ˮ��������CCl4��ʵ�����Ʊ�1-���������װ����ͼ��ʾ��
1-��������[��CH3��2CHCH2CH2Br]���л��ϳɵ���Ҫ�м��壬����������Ⱦ�ϡ������ȣ���е�Ϊ121�棬������CCl4���������촼��������������������·�Ӧ���ã���CH3��2CHCH2CH2OH+HBr$\stackrel{��}{��}$��CH3��2CHCH2CH2Br+H2O����֪���촼�ķе�Ϊ132.5�棬����ˮ��������CCl4��ʵ�����Ʊ�1-���������װ����ͼ��ʾ����ش��������⣺
��1������ʵ��װ���г����ܵ���������������������ĩ�˲���CCl4�ж���ֱ�Ӳ���ˮ�У������ܽ��ӷ�����Br2��1-��������ȳ�������⣬��һ����Ҫ�����Ƿ�ֹ������
��2����1-��������Ĵֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã��л��ォ���²㣨����ϲ㡱�������²㡱���ֲ㡱����
��3�����Ʊ������У�Br-�ɱ�����ΪBr2���������ʣ�����ȥBr2����ѡ��CE������ĸ����
A��NaI������B��NaOH������C��NaHSO3������D��KCl������E��NaHCO3
��4�����Ʊ�1-��������ʱ�����ܱ߷�Ӧ�����������ԭ����1-�嶡�����������ķе���С�����߷�Ӧ������������н϶���������ӷ�������ԭ�ϵ������ʣ�
��5����ʵ�����л�����NaBr��ŨH2SO4�����촼Ϊԭ���Ʊ�1-�������飮��֪��Ӧ����������±���
| ��Ӧ�� | NaBr | 98.3% ŨH2SO4 | ���촼 | ˮ |
| ���� | 0.30mol | 35mL�������� | 0.25mol | 30mL |
| A�� | MnO2����������Ӧ��HCl������ԭ��Ӧ | |
| B�� | ÿ��Ӧ��4mol HClת��2 mol���� | |
| C�� | ÿ����1mol Cl2ת��4 mol���� | |
| D�� | ��Ӧ����Ԫ�ؽ�����ת�Ƹ���Ԫ�� |
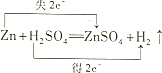

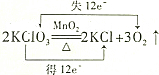



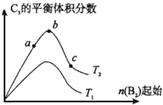 ��֪2A2��g��+B2��g��?2C3��g������H=-Q1kJ/mol��Q1��0������һ���д����Ĺ̶��ݻ��������м���2molA2��1molB2����500��ʱ��ַ�Ӧ����ƽ���C3��Ũ��Ϊw mol•L-1���ų�����ΪQ2kJ��
��֪2A2��g��+B2��g��?2C3��g������H=-Q1kJ/mol��Q1��0������һ���д����Ĺ̶��ݻ��������м���2molA2��1molB2����500��ʱ��ַ�Ӧ����ƽ���C3��Ũ��Ϊw mol•L-1���ų�����ΪQ2kJ��