��Ŀ����
(1)ͬ��ͬѹ�£�ͬ�����NH3��H2S�������������___________��ͬ������NH3��H2S������������__________��ͬ������NH3��H2S������������ԭ�Ӹ�������___________��������������ԭ�Ӹ�����ȣ����ǵ����ʵ�������________��
(2)Na2SO4??10H2O��Ħ��������__________��483gNa2SO4??10H2O������Na2SO4??10H2O�����ʵ�����_______������Na�������ʵ�����_________������H2O���ӵ���Ŀ��_______����
(2)Na2SO4??10H2O��Ħ��������__________��483gNa2SO4??10H2O������Na2SO4??10H2O�����ʵ�����_______������Na�������ʵ�����_________������H2O���ӵ���Ŀ��_______����
��ÿ��1�֣���8�֣� ��1��1:2 ��2:1 ��3:1 �� 2:3��2��322g/mol��1.5mol�� 3 mol ��9.03��1024
�����������1�����ݰ����ӵ����ɿ�֪��ͬ��ͬѹ�£�ͬ�����NH3��H2S�������ʵ�����ȣ������������17:34��1:2��ͬ������NH3��H2S������������
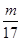 :
: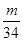 ��2:1��ͬ������NH3��H2S������������ԭ�Ӹ�������
��2:1��ͬ������NH3��H2S������������ԭ�Ӹ�������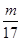 ��3:
��3: 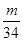 ��2��3:1��������������ԭ�Ӹ�����ȣ�����ݷ���ʽ��֪�����ǵ����ʵ�����2:3��
��2��3:1��������������ԭ�Ӹ�����ȣ�����ݷ���ʽ��֪�����ǵ����ʵ�����2:3����2��Na2SO4??10H2O��Ħ�������ǣ�142+180��g/mol��322g/mol ��483gNa2SO4??10H2O������Na2SO4
??10H2O�����ʵ�����483g��322g/mol��1.5mol������Na�������ʵ�����1.5mol��2��3.0mol������H2O���ӵ���Ŀ��1.5mol��10��6.02��1023/mol9.03��1024����

��ϰ��ϵ�д�
 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�
�����Ŀ
 ����ȡ����������17.4g
����ȡ����������17.4g ��ȫ��Ӧʱ�����㣺
��ȫ��Ӧʱ�����㣺 �����ʵ�����
�����ʵ�����