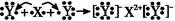��Ŀ����
X��Y��Z��W���ֶ�����Ԫ����Ԫ�����ڱ���λ����ͼ������W��ԭ��������Y��2����
��1��W���ӵĽṹʾ��ͼΪ____________��
��2��X������⻯����Cl2����ȡ����Ӧ�Ļ�ѧ����ʽΪ(дһ��)________________��
��3����֤��X��Z��Ԫ�طǽ�����ǿ�������ӷ���ʽΪ__________________________��
��4��һ�������£����ܱ������У�����һ������XY2��g����XY (g)��������Ӧ��
WY2(g)+2XY(g)  2XY2 (g)+W��1����T1oCʱ�������ʵ�ƽ��Ũ�����±���
2XY2 (g)+W��1����T1oCʱ�������ʵ�ƽ��Ũ�����±���
�����¶����ߵ�T2oCʱ����Ӧ��ƽ�ⳣ��Ϊ6.64����÷�Ӧ������ӦΪ_______��Ӧ(�� �����ȡ����ȡ�)��
��5���� 25�桢101 kPa��l mol����þ��ȫȼ�շų�300.0 kJ������1 mol����X��ȫȼ�շų�393.5 kJ����������þ��X����������ﷴӦ���Ȼ�ѧ����ʽΪ_________��
�������£�Mg(OH) 2������Һ_________(��ܡ����ܡ�)ʹ��̪��Һ��죬ͨ������˵��ԭ��(��֪��Ksp[Mg(OH) 2]=4.0��10-12)����__________________________
��1��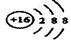
��2��CH4 + Cl2 �� CH3Cl+HCl
��3��CO2+SiO32��+H2O= CO32��+H2SiO3��
��4������
��5��2Mg(s)+ CO2(g) =" 2MgO(s)+C(s)" ��H=-206.5kJ/mol
��6���� Ksp[Mg(OH)2]=4.0��10-12=c(Mg2+)��c2(OH��)
1/2c3(OH��)= 4.0��10-12
c(OH��) =2.0��10-4
pOH=3.7
pH=14��3.7=10.3
��̪�ı�ɫ��ΧΪ8.2����10���ʱ�졣
�������������W��Yͬ���壬��W��ԭ��������Y��2����ֻ��O��S���ϣ�X��Y��Z��W�ֱ�ΪC��O��Si��S����2��X������⻯��Ϊ���飬��������������ȡ����Ӧ������CH3Cl��CH2Cl2��CHCl3��CCl4����3���Ƚ�Ԫ�طǽ�����ǿ�����Ը�������������Ӧˮ�������ԡ����������ϵ��������⻯���ȶ��Լ����ʵ��û���Ӧ����֤��X��Z��Ԫ�طǽ�����ǿ���ķ�Ӧ���Դ�ǿ��������ͳ��������µĵ����û���Ӧ����������ɸ���̼�����ɹ���ó����ۣ�
��4����������ƽ�ⳣ��k=c2(XY2)/ c2(XY) c(WY2)=4.44��6.64�������¶�ƽ�������ƶ�����Ӧ���ȣ�
��5����Mg(s)+1/2O2(g)=" MgO(s)" ��H="-300.0" kJ/mol
��C(s)+O2(g)= CO2(g) ��H="-393.5" kJ/mol
�١�2���ڵó�2Mg(s)+ CO2(g) =" 2MgO(s)+C(s)" ��H=-206.5kJ/mol
��Ksp[Mg(OH)2]=4.0��10-12=c(Mg2+)��c2(OH��) 1/2c3(OH��)= 4.0��10-12 c(OH��) =2.0��10-4
pOH=3.7 pH=14��3.7=10.3 ��̪�ı�ɫ��ΧΪ8.2����10���ʱ�졣
���㣺���黯ѧ�ۺ��漰ԭ�ӽṹ�������ɡ�ƽ���ƶ����Ȼ�ѧ���ܶȻ�������й����⡣

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�A��B��C��D��E�����ڱ���ǰ�����ڵ�Ԫ��,���й����ʻ�ṹ��Ϣ���±�:
| Ԫ�� | �й����ʻ�ṹ��Ϣ |
| A | �����۵�AԪ�ص��⻯����ͨ��״������һ��Һ��,����A����������Ϊ88.9% |
| B | Bԭ�ӵõ�һ�����Ӻ�3p���ȫ���� |
| C | Cԭ�ӵ�p��������,������̬�⻯������������������ˮ���ﷴӦ����һ�ֳ�������X |
| D | DԪ�ص�����ϼ�����ͻ��ϼ۵Ĵ�����Ϊ��,�����������Ϊ���Ӿ��� |
| E | EԪ�صĺ˵��������AԪ�غ�BԪ���⻯��ĺ˵����֮�� |
(1)Ԫ��Y��C��һ����ͬ����Ԫ��,�Ƚ�B��YԪ�صĵ�һ������I1(B)��������I1(Y)��
(2)EԪ��ԭ�ӵĺ�������Ų�ʽΪ��
(3)��X��ˮ��Һ����������(����ԡ��������ԡ������ԡ�),BԪ����ۺ�����һ����DԪ����ۺ������������������(�ǿ��������)��
(4)C���ʷ����ЦҼ��ͦм��ĸ�����Ϊ��������,C���⻯����ͬ��Ԫ�ص��⻯���зе���ַ���,��ԭ������ ��
(5)�ø�����������Һ̬H2Aʱ,һ��H2A�������ͷų�һ������,ͬʱ����һ�־��н�ǿ�����Ե�������,��д���������ӵĵ���ʽ:�� ,
д����������������⻯���ˮ��Һ��Ӧ�����ӷ���ʽ:
������Ԫ��A��X��D��E��R��Tԭ��������������ԭ�ӽṹ�����������ʾ��
| Ԫ�� | �ṹ������ |
| A | A��ԭ�Ӱ뾶��С |
| X | Xԭ�������������Ǵ��������� |
| D | D�Ƕ������н�������ǿ��Ԫ�� |
| E | E������������Ӧˮ������һ�ֳ����������������� |
| R | R��Xͬ���� |
| T | T�ĸ�һ�������ӵĺ�������Ų���Arԭ����ͬ |
��1��RԪ�������ڱ���λ���� ��������DT�д��ڵĻ�ѧ���� ��
��2��д��E������NaOH��Һ��Ӧ�����ӷ���ʽ ��
��3��1g X2A4 ��ȫȼ�գ��ָ�������ʱ�ų�a kJ��������д��X2A4��ȫȼ�յ��Ȼ�ѧ����ʽ ��
��4��RT4����ˮ�����������ᣬд���÷�Ӧ�Ļ�ѧ����ʽ ��
��5����֪ij�¶���T��ijһԪ�������Ka = 4��0��10-8����һ��Ũ�ȸ����pH=4�������Һ�����ʵ���Ũ��Ϊ ��
���й���Ԫ�������ɺ�Ԫ�����ڱ�����������ȷ����(����)��
| A��ͬһ�����Ԫ�ش��ϵ��½��������� |
| B��Ԫ�����ڱ���Ԫ�������ɵľ��������ʽ |
| C��ͬһ���ڴ����ң�Ԫ��ԭ�Ӱ뾶������ |
| D���ǽ���Ԫ�ص���������ϼ۵������ĸ����ϼ۵ľ���ֵ |
 ����֪��Ԫ�ص�ԭ�Ӻ�������������������ȣ����Ԫ�ص�������________��
����֪��Ԫ�ص�ԭ�Ӻ�������������������ȣ����Ԫ�ص�������________��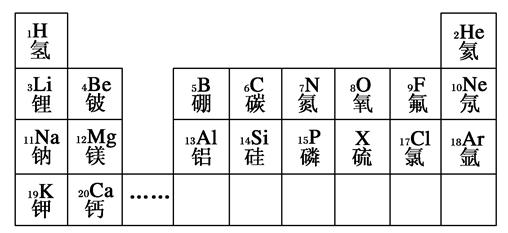
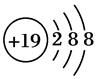 ��ʾ���ǣ�д���ӷ��ţ�________��
��ʾ���ǣ�д���ӷ��ţ�________��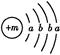 ,X����������Y�������ӵĵ��Ӳ�ṹ��ͬ��Ԫ��Z��W��Ϊ������Ԫ��,����ԭ�ӵ�������������������Ӳ�����2��,Z��Y������Z��W���γ�һ��WZ2�ͷ��ӡ�
,X����������Y�������ӵĵ��Ӳ�ṹ��ͬ��Ԫ��Z��W��Ϊ������Ԫ��,����ԭ�ӵ�������������������Ӳ�����2��,Z��Y������Z��W���γ�һ��WZ2�ͷ��ӡ�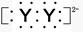
 2Y-+Z��
2Y-+Z��