题目内容
【题目】25℃时,有c(CH3COOH)+c(CH3COO-)=0.1mol/L的一组CH3COOH、CH3COONa的混合溶液,溶液中c(CH3COOH)、c(CH3COO-)与pH的关系如图所示.下列有关溶液中离子浓度关系的叙述正确的是( )
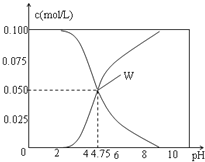
A. pH=3.5的溶液中:c(Na+)+c(H+)-c(OH-)+c(CH3COOH)=0.1mol/L
B. pH=5.5的溶液中:c(CH3COOH)>c(CH3COO-)>c(H+)>c(OH-)
C. W点所表示的溶液中:c(H+)=c(CH3COOH)+c(OH-)
D. 向W点所表示的1.0L溶液中通入0.05molHCl气体(溶液体积变化可忽略):c(H+)=c(CH3COOH)+c(OH-)
【答案】A
【解析】CH3COOH、CH3COONa的混合溶液中的电荷守恒为c(Na+)+c(H+)=c(CH3COO-)+c(OH-)。A,c(Na+)+c(H+)-c(OH-)+c(CH3COOH)=c(CH3COO-)+c(CH3COOH)=0.1mol/L,A项正确;B,由图像知pH=4.75时c(CH3COO-)=c(CH3COOH),pH=5.5时溶液中c(CH3COO-)![]() c(CH3COOH),B项错误;C,根据电荷守恒c(H+)= c(CH3COO-)+c(OH-)-c(Na+),由图知W点溶液中c(CH3COOH)=c(CH3COO-),则c(H+)= c(CH3COOH)+c(OH-)-c(Na+),C项错误;D,W点溶液中c(CH3COOH)=c(CH3COO-)=0.05mol/L,1LW点溶液中n(CH3COO-)=0.05mol,通入0.05molHCl,CH3COO-与H+恰好完全反应生成CH3COOH,由于CH3COOH的电离是微弱的,c(CH3COOH)
c(CH3COOH),B项错误;C,根据电荷守恒c(H+)= c(CH3COO-)+c(OH-)-c(Na+),由图知W点溶液中c(CH3COOH)=c(CH3COO-),则c(H+)= c(CH3COOH)+c(OH-)-c(Na+),C项错误;D,W点溶液中c(CH3COOH)=c(CH3COO-)=0.05mol/L,1LW点溶液中n(CH3COO-)=0.05mol,通入0.05molHCl,CH3COO-与H+恰好完全反应生成CH3COOH,由于CH3COOH的电离是微弱的,c(CH3COOH)![]() c(H+),D项错误;答案选A。
c(H+),D项错误;答案选A。