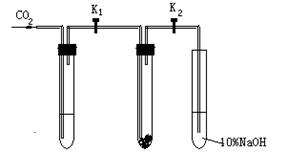
��Ŀ����
Ϊ��̽����������̼�Ƿ�ֻ����ˮ����ʱ���ܺ������Ʒ�Ӧ����ij�����о�С���ͬѧ�����������ͼ��ʾ��ʵ��װ�ã��ֱ���мס�������ʵ�顣
ʵ��ף�����Ķ�����̼�������Ƶķ�Ӧ
�ڸ�����Թܢ���װ��Na2O2����ͨ��CO2֮ǰ���ر�K1��K2�����Թܢ���װ���Լ�X��K1��K2��ͨ��CO2�������Ӻ������ǵ�ľ���쵽�Թܢ��Һ���ϣ��۲쵽ľ��û�и�ȼ�����Թܢ��е���ɫ����û�з����仯��
ʵ���ң���ʪ�Ķ�����̼�������Ƶķ�Ӧ
���Թܢ���װ���Լ�Y����������ͬʵ��ף��۲쵽ľ����ȼ�����Թܢ��е���ɫ�����Ϊ��ɫ��
�Իش��������⣺
��1����װ��Na2O2��ͨ��CO2ǰ���ر�K1��K2��Ŀ����___________________________________��
��2����ʵ����У��Լ�X��__________����������_________����ʵ�����У��Լ�Y��________��
��3��������������ʵ������õ��Ľ�����_________________________________________��
��4���Թܢ��е�NaOH��Һ��������________
________________________��
��5��Ϊ��ȷ��ʵ�������ȷ�ԣ��Ʊ�CO2���õķ�Ӧ�����ѡ��______�����ţ���
A.����ʯ B.С�մ� C.�ռ�
D.���� E.ϡ���� F.ϡ����
��6��CO2��Na2O2�ķ�Ӧ��������ʾ��ԭ�ӷ���������֤������������з�Ӧ��
_____Na2O2+_____C18O2+_____H218O=__________
��1����ֹˮ���������Թܢ��У�ʹNa2O2�ܳ���Ӱ��ʵ����
��2��Ũ���� ��ȥ������̼�е�ˮ���� CO2�ı�����Һ����ˮ��
��3��������ֻ̼����ˮ����ʱ���ܺ������Ʒ�Ӧ
��4����ȥ���������еĶ�����̼
��5��BE
��6��2Na2O2+2C18O2+2H218O=2Na2C18O3+O2+2H2O
�����������
�����������ʵ��Ŀ����̽����������̼�Ƿ�ֻ����ˮ����ʱ���ܺ������Ʒ�Ӧ��������ڽ���ʵ���ʱӦ��֤Na2O2���Բ��Ӵ�ˮ����������ͨ��CO2ǰ�ر�K1��K2��ԭ���Լ�XҲ�������CO2�����á�ʵ���������෴������ʹ�Ӵ�Na2O2��CO2�л���ˮ����������Լ�YӦ�Dz�������ˮ�������������ṩˮ���������ʡ�
��ʪ��CO2ͨ��Na2O2ʱ����Ӧ������O2����CO2��һ���ܳ�ַ�Ӧ�������Թܢ��г�����������CO2����������ϴ�ʱ�����Թܢ��м���O2��������������������Ҫ��NaOH��Һ����O2�е�CO2��
��ΪNa2O2�ܺ��ᷴӦ�������Ʊ�CO2���õ���Ӧ���Dz��ӷ����ᣬѡ���е�ϡ��������Ҫ����Ӧ���ξ�ֻ����NaHCO3�ˡ�

��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ
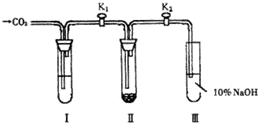 Ϊ��̽����������̼�Ƿ���ˮ����ʱ���ܺ������Ʒ�Ӧ���������о�С���ͬѧ���������ͼ��ʾ��ʵ��װ�ã��ֱ���мס�������ʵ�飺
Ϊ��̽����������̼�Ƿ���ˮ����ʱ���ܺ������Ʒ�Ӧ���������о�С���ͬѧ���������ͼ��ʾ��ʵ��װ�ã��ֱ���мס�������ʵ�飺